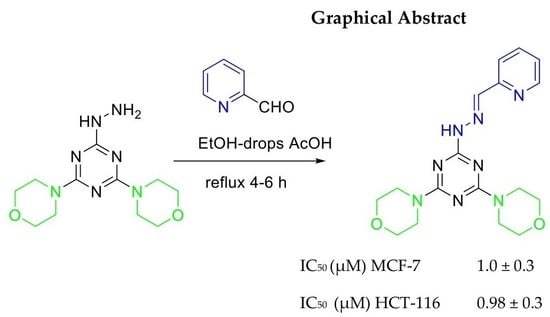
Synthesis and Characterization of New Series of 1,3-5-Triazine Hydrazone Derivatives with Promising Antiproliferative Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biology
2.2.1. Antiproliferative Activity
3. Experimental Section
3.1. Materials and Methods
3.2. General Method for the Synthesis of 1,3,5-Triazine-Hydrazone Derivatives
3.2.1. 2-(2-Benzylidenehydrazinyl)-4,6-di(piperidin-1-yl)-1,3,5-triazine, 7a
3.2.2. 2-(2-(4-Chlorobenzylidene)hydrazinyl)-4,6-di(piperidin-1-yl)-1,3,5-triazine, 7b
3.2.3. 2-(2-(4-Bromobenzylidene)hydrazinyl)-4,6-di(piperidin-1-yl)-1,3,5-triazine, 7c
3.2.4. 4-((2-(4,6-Di(piperidin-1-yl)-1,3,5-triazin-2-yl)hydrazono)methyl)phenol, 7d
3.2.5. 2-(2-(4-Fluorobenzylidene)hydrazinyl)-4,6-di(piperidin-1-yl)-1,3,5-triazine, 7e
3.2.6. 2-(2-(4-Methoxybenzylidene)hydrazinyl)-4,6-di(piperidin-1-yl)-1,3,5-triazine, 7f
3.2.7. 4,4’-(6-(2-(4-Fluorobenzylidene)hydrazinyl)-1,3,5-triazine-2,4-diyl)dimorpholine, 8e
3.2.8. 4-(4-(2-Benzylidenehydrazinyl)-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)morpholine, 9a
3.2.9. 4-(4-(2-(4-Chlorobenzylidene)hydrazinyl)-6-(piperidin-1-yl)-1,3,5-triazin-2- yl)morpholine, 9b
3.2.10. 4-(4-(2-(4-Bromobenzylidene)hydrazinyl)-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)morpholine, 9c
3.2.11. 4-((2-(4-Morpholino-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)hydrazono)methyl)phenol, 9d
3.2.12. 4-(4-(2-(4-Fluorobenzylidene)hydrazinyl)-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)morpholine, 9e
3.2.13. 4-(4-(2-(4-Methoxybenzylidene)hydrazinyl)-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)morpholine, 9f
3.2.14. 2,4-Di(piperidin-1-yl)-6-(2-(pyridin-2-ylmethylene)hydrazinyl)-1,3,5-triazine, 10
3.2.15. 4,4’-(6-(2-(Pyridin-2-ylmethylene)hydrazinyl)-1,3,5-triazine-2,4-diyl)dimorpholine, 11
3.2.16. 4-(4-(Piperidin-1-yl)-6-(2-(pyridin-2-ylmethylene)hydrazinyl)-1,3,5-triazin-2-yl)morpholine, 12
3.3. Biology
3.3.1. In Vitro Antiproliferative Assay
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharma, A.; El-Faham, A.; de la Torre, B.G.; Albericio, F. Exploring the orthogonal chemoselectivity of 2,4,6-trichloro-1,3,5-triazine (TCT) as a trifunctional linker with different nucleophiles: Rules of the game. Front. Chem. 2018, 6, 516. [Google Scholar] [CrossRef] [Green Version]
- Huy, P.H.; Mbouhom., C. Formamide catalyzed activation of carboxylic acids–versatile and cost-efficient amidation and esterification. Chem. Sci. 2019, 10, 7399–7406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blotny, G. Recent applications of 2, 4, 6-trichloro-1, 3, 5-triazine and its derivatives in organic synthesis. Tetrahedron 2006, 62, 9507–9522. [Google Scholar] [CrossRef]
- Zhou, C.; Min, J.; Liu, Z.; Young, A.; Deshazer, H.; Gao, T.; Kallenbach, N.R. Synthesis and biological evaluation of novel 1, 3, 5-triazine derivatives as antimicrobial agents. Bioorg. Med. Chem. Lett. 2008, 18, 1308–1311. [Google Scholar] [CrossRef] [PubMed]
- Bahar, A.A.; Liu, Z.; Garafalo, M.; Kallenbach, N.; Ren, D. Controlling persister and biofilm cells of gram-negative bacteria with a new 1, 3, 5-triazine derivative. Pharmaceuticals 2015, 8, 696–710. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, K.N.; Sarmah, N.K.; Kurmi, K.B.; Patel, T.V. Synthesis and studies of antifungal activity of 2, 4, 6-trisubstituted 1, 3, 5-triazines. Adv. Appl. Sci. Res. 2012, 3, 1459–1462. [Google Scholar]
- Ng, H.L.; Ma, X.; Chew, E.H.; Chui, W.K. Design, synthesis, and biological evaluation of coupled bioactive scaffolds as potential anticancer agents for dual targeting of dihydrofolate reductase and thioredoxin reductase. J. Med. Chem. 2017, 60, 1734–1745. [Google Scholar] [CrossRef]
- Nishimura, N.; Kato, A.; Isamu, M. Synthesis of pyrrolo[2,1-f][1,2,4]triazine C-nucleosides. Isosteres of sangivamycin, tubercidin, and toyocamycin. Carbohydr. Res. 2001, 331, 77–82. [Google Scholar] [CrossRef]
- Zacharie, B.; Abbott, S.D.; Bienvenu, J.F.; Cameron, A.D.; Cloutier, J.; Duceppe, J.S.; Ezzitouni, A.; Fortin, D.; Houde, K.; Lauzon, C. 2, 4, 6-trisubstituted triazines as protein a mimetics for the treatment of autoimmune diseases. J. Med. Chem. 2010, 53, 1138–1145. [Google Scholar] [CrossRef]
- Klenke, B.; Stewart, M.; Barrett, M.P.; Brun, R.; Gilbert, I.H. Synthesis and biological evaluation of s-triazine substituted polyamines as potential new anti-trypanosomal drugs. J. Med. Chem. 2001, 44, 3440–3452. [Google Scholar] [CrossRef]
- Mibu, N.; Yokomizo, K.; Aki, H.; Ota, N.; Fujii, H.; Yuzuriha, A.; Saneyoshi, S.; Tanaka, A.; Koga, A.; Zhou, J. Synthesis and antiviral evaluation of some C3-symmetrical trialkoxy substituted 1, 3, 5-triazines and their molecular geometry. Chem. Pharm. Bull. 2015, 63, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.R.; Modh, R.P.; Chikhalia, K.H. Privileged s-triazines: Structure and pharmacological applications. Future. Med. Chem. 2014, 6, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Sun, T.; Zhou, Z.; Du, L. A systematic review on antitumor agents with 1, 3, 5-triazines. Med. Chem. 2015, 5, 131–148. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Liu, Y.; Cui, Z.; Beattie, D.; Gu, Y.; Wang, Q. Design, synthesis, and biological activities of arylmethylamine substituted chlorotriazine and methylthiotriazine compounds. J. Agric. Food. Chem. 2011, 59, 11711–11717. [Google Scholar] [CrossRef]
- Hu, Z.; Ma, T.; Chen, Z.; Ye, Z.; Zhang, G.; Lou, Y.; Yu, Y. Solid-phase synthesis and antitumor evaluation of 2, 4-diamino-6-aryl-1, 3, 5-triazines. J. Comb. Chem. 2009, 11, 267–273. [Google Scholar] [CrossRef]
- Zheng, M.; Xu, C.; Ma, J.; Sun, Y.; Du, F.; Liu, H.; Lin, L.; Li, C.; Ding, J.; Chen, K. Synthesis and antitumor evaluation of a novel series of triamino-triazine derivatives. Bioorg. Med. Chem. 2007, 15, 1815–1827. [Google Scholar] [CrossRef]
- Sączewski, F.; Bułakowska, A.; Bednarski, P.; Grunert, R. Synthesis, structure and anticancer activity of novel 2, 4-diamino-1, 3, 5-triazine derivatives. Eur. J. Med. Chem. 2006, 41, 219–225. [Google Scholar] [CrossRef]
- Sączewski, F.; Bułakowska, A. Synthesis, structure and anticancer activity of novel alkenyl-1, 3, 5-triazine derivatives. Eur. J. Med. Chem. 2006, 41, 611–615. [Google Scholar] [CrossRef]
- Perspicace, E.; Jouan-Hureaux, V.; Ragno, R.; Ballante, F.; Sartini, S.; La Motta, C.; Da Settimo, F.; Chen, B.; Kirsch, G.; Schneider, S. Design, synthesis and biological evaluation of new classes of thieno [3, 2-d] pyrimidinone and thieno [1,2,3] triazine as inhibitor of vascular endothelial growth factor receptor-2 (VEGFR-2). Eur. J. Med. Chem. 2013, 63, 765–781. [Google Scholar] [CrossRef]
- Srivastava, G.K.; Alonso-Alonso, M.L.; Fernandez-Bueno, I.; Garcia-Gutierrez, M.T.; Rull, F.; Medina, J.; Coco, R.M.; Pastor, J.C. Comparison between direct contact and extract exposure methods for PFO cytotoxicity evaluation. Sci. Rep. 2018, 8, 1425. [Google Scholar] [CrossRef]
- Menear, K.A.; Gomez, S.; Malagu, K.; Bailey, C.; Blackburn, K.; Cockcroft, X.L.; Sebastian, L.; Ewen, S.; Fundo, A.; le Gall, A.; et al. Identification and optimisation of novel and selective small molecular weight kinase inhibitors of mTOR. Bioorg. Med. Chem. Lett. 2009, 19, 5898–5901. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Liu, H.; Tong, L.; Zhou, W.; Liu, L.; Zhao, Z.; Liu, X.; Jiang, H.; Wang, X.; Xie, H. Discovery of novel selective inhibitors for EGFR-T790M/L858R. Bioorg. Med. Chem. Lett. 2012, 22, 1365–1370. [Google Scholar] [CrossRef] [PubMed]
- El-Faham, A.; Soliman, S.M.; Ghabbour, H.A.; Elnakady, Y.A.; Mohaya, T.A.; Siddiqui, M.R.; Albericio, F. Ultrasonic promoted synthesis of novel s-triazine-Schiff base derivatives; molecular structure, spectroscopic studies and their preliminary anti-proliferative activities. J. Mol. Str. 2016, 1125, 121–135. [Google Scholar] [CrossRef]
- Barakat, A.; El-Senduny, F.F.; Almarhoon, Z.; Al-Rasheed, H.H.; Badria, F.A.; Al-Majid, A.M.; El-Faham, A. Synthesis, X-Ray Crystal Structures, and Preliminary Antiproliferative Activities of New s-Triazine-hydroxybenzylidene Hydrazone Derivatives. J. Chem. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Kumari, P.; Singh, G.; Bhatti, R.; Singh, P. Design and synthesis of aza-/oxa heterocycle-based conjugates as novel anti-Inflammatory agents targeting cyclooxygenase-2. ACS Omega 2018, 3, 5825–5845. [Google Scholar] [CrossRef]
- Sharma, A.; Ghabbour, H.; Khan, S.T.; Beatriz, G.; Albericio, F.; El-Faham, A. Novel pyrazolyl-s-triazine derivatives, molecular structure and antimicrobial activity. J. Mol. Str. 2017, 1145, 244–253. [Google Scholar] [CrossRef]
- El-Faham, A.; Elnakady, Y. Synthesis, characterization of novel morpholino-1, 3, 5-triazinyl amino acid Ester derivatives and their anti-proliferation activities. Lett. Org. Chem. 2015, 12, 753–758. [Google Scholar] [CrossRef]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donnelly, D.J.; Meanwell, N.A. Applications of fluorine in medicinal chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef]
- Swallow, S. Fluorine in medicinal chemistry. In Progress in Medicinal Chemistry, 1st ed.; Witty, D.R., Lawton, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 54, pp. 65–133. [Google Scholar] [CrossRef]
- Al-Rasheed, H.H.; Al Alshaikh, M.; Khaled, J.M.; Alharbi, N.S.; El-Faham, A. Ultrasonic irradiation: Synthesis, characterization, and preliminary antimicrobial activity of novel series of 4, 6-disubstituted-1, 3, 5-triazine containing hydrazone derivatives. J. Chem. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds.are not available from the authors. |



| Cpd No. | R1 | R2 | X | Cpd No. | R1 | R2 | X |
|---|---|---|---|---|---|---|---|
| 7a | piperidine | piperidine | H | 9a | morpholine | piperidine | H |
| 7b | piperidine | piperidine | Cl | 9b | morpholine | piperidine | Cl |
| 7c | piperidine | piperidine | Br | 9c | morpholine | piperidine | Br |
| 7d | piperidine | piperidine | OH | 9d | morpholine | piperidine | OH |
| 7e | piperidine | piperidine | F | 9e | morpholine | piperidine | F |
| 7f | piperidine | piperidine | OCH3 | 9f | morpholine | piperidine | OCH3 |
| 8a | morpholine | morpholine | H | 10 | piperidine | piperidine | |
| 8b | morpholine | morpholine | Cl | 11 | morpholine | morpholine | |
| 8c | morpholine | morpholine | Br | 12 | morpholine | piperidine | |
| 8d | morpholine | morpholine | OH | ||||
| 8e | morpholine | morpholine | F |
| Compound No. | IC50 (μM) MCF-7 | IC50 (μM) HCT-116 |
|---|---|---|
| 7a | 17.5 ± 5.9 | 12.6 ± 4.6 |
| 7b | 23.5 ± 8.0 | 10.8 ± 3.5 |
| 7c | 39.8 ± 12.9 | 10.9 ± 3.6 |
| 7d | 18.2 ± 5.8 | 19.5 ± 6.2 |
| 7e | 11.5 ± 3.3 | 14.0 ± 4.4 |
| 7f | 39.9 ± 12.7 | 8.8 ± 2.5 |
| 8a | 29.3 ± 9.3 | >50 |
| 8b | 17.6 ± 5.7 | 19.2 ± 6.2 |
| 8c | 23.2 ± 7.3 | 38.7 ± 12.4 |
| 8d | 14.0 ± 4.8 | 29.9 ± 9.5 |
| 8e | 13.4 ± 4.0 | 18.3 ± 5.7 |
| 9a | 22.2 ± 7.9 | 44.2 ± 14.4 |
| 9b | 21.9 ± 6.0 | 30.0 ± 9.6 |
| 9c | 22.5 ± 3.9 | 28.2 ± 9.0 |
| 9d | 10.4 ± 3.1 | 25.4 ± 8.2 |
| 9e | 13.9 ± 4.7 | 22.0 ± 7.1 |
| 9f | 14.2 ± 4.5 | 23.4 ± 7.4 |
| 10 | 32.8 ± 10.4 | >50 |
| 11 | 1.0 ± 0.3 | 0.98 ± 0.3 |
| 12 | 17.7 ± 5.5 | 30.4 ± 9.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
H. Al Rasheed, H.H.A.; M. Malebari, A.M.; A. Dahlous, K.A.; El-Faham, A. Synthesis and Characterization of New Series of 1,3-5-Triazine Hydrazone Derivatives with Promising Antiproliferative Activity. Molecules 2020, 25, 2708. https://doi.org/10.3390/molecules25112708
H. Al Rasheed HHA, M. Malebari AM, A. Dahlous KA, El-Faham A. Synthesis and Characterization of New Series of 1,3-5-Triazine Hydrazone Derivatives with Promising Antiproliferative Activity. Molecules. 2020; 25(11):2708. https://doi.org/10.3390/molecules25112708
Chicago/Turabian StyleH. Al Rasheed, Hessa H. Al, Azizah M. M. Malebari, Kholood A. A. Dahlous, and Ayman El-Faham. 2020. "Synthesis and Characterization of New Series of 1,3-5-Triazine Hydrazone Derivatives with Promising Antiproliferative Activity" Molecules 25, no. 11: 2708. https://doi.org/10.3390/molecules25112708
APA StyleH. Al Rasheed, H. H. A., M. Malebari, A. M., A. Dahlous, K. A., & El-Faham, A. (2020). Synthesis and Characterization of New Series of 1,3-5-Triazine Hydrazone Derivatives with Promising Antiproliferative Activity. Molecules, 25(11), 2708. https://doi.org/10.3390/molecules25112708