Stabilisation of Exotic Tribromide (Br3−) Anions via Supramolecular Interaction with a Tosylated Macrocyclic Pyridinophane. A Serendipitous Case
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystallographic Results
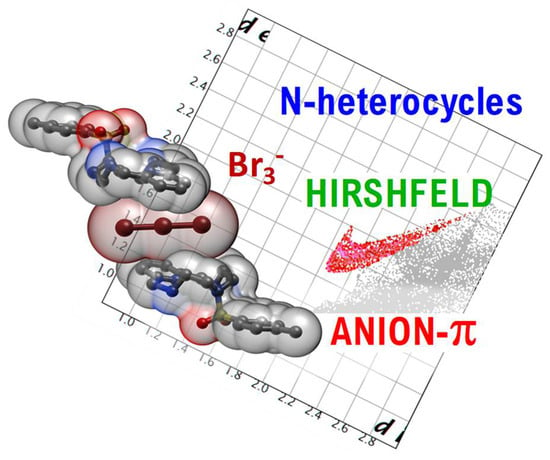
2.2. Detecting Anion-π Interaction from Fingerprint Plots
2.2.1. Relevance and Method
2.2.2. Analysis
3. Materials and Methods
3.1. General
3.2. Synthesis of (H2L-Ts)(Br3)1.5(NO3)0.5 (1)
3.3. X-ray Structure Analyses
3.4. Hirshfeld Surface Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Donnio, B.; Guillon, D.; Deschenaux, R.; Bruce, D.W. Comprehensive Coordination Chemistry II; McCleverty, J.A., Meyer, T.J., Lever, A.B.P., Eds.; Elsevier: Oxford, UK, 2003; Volume 1. [Google Scholar]
- Bowman-James, K.; Bianchi, A.; García-España, E. (Eds.) Anion Coordination Chemistry; Wiley-VCH: New York, NY, USA, 2012. [Google Scholar]
- Sessler, J.L.; Gale, P.A.; Cho, W.S. Anion Receptor; RSC Publishing: Cambridge, UK, 2006. [Google Scholar]
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry, 2nd ed.; John Wiley & Sons: Chichester, UK, 2009. [Google Scholar]
- Stetter, H.; Frank, W.; Mertens, R. Darstellung und komplexbildung von polyazacycloalkan-N-essigsäuren. Tetrahedron 1981, 37, 767–772. [Google Scholar] [CrossRef]
- Costa, J.; Delgado, R. Metal complexes of macrocyclic ligands containing pyridine. Inorg. Chem. 1993, 32, 5257–5265. [Google Scholar] [CrossRef]
- Félix, V.; Costa, J.; Delgado, R.; Drew, M.G.B.; Duarte, M.T.; Resende, C. X-Ray diffraction and molecular mechanics studies of 12-, 13-, and 14-membered tetraaza macrocycles containing pyridine: Effect of the macrocyclic cavity size on the selectivity of the metal ion. J. Chem. Soc. Dalton Trans. 2001, 1462–1471. [Google Scholar] [CrossRef]
- Brewer, S.M.; Wilson, K.R.; Jones, D.G.; Reinheimer, E.W.; Archibald, S.J.; Prior, T.J.; Ayala, M.A.; Foster, A.L.; Hubin, T.J.; Green, K.N. Increase of direct C-C coupling reaction yield by identifying structural and electronic properties of high-spin iron tetra-azamacrocyclic complexes. Inorg. Chem. 2018, 57, 8890–8902. [Google Scholar] [CrossRef] [PubMed]
- Frías, J.C.; Soriano, J.; Blasco, S.; García-España, E.; Rodríguez-Rodríguez, A.; Esteban-Gomez, D.; Carniato, F.; Botta, M.; Platas-Iglesias, C.; Albelda, M.T. Macrocyclic pyclen-based Gd3+ complex with high relaxivity and pH response. Inorg. Chem. 2020, 59, 7306–7317. [Google Scholar] [CrossRef] [PubMed]
- Tircso, G.; Kovacs, Z.; Sherry, A.D. Equilibrium and Formation/Dissociation Kinetics of Some LnIIIPCTA Complexes. Inorg. Chem. 2006, 45, 9269–9280. [Google Scholar] [CrossRef] [Green Version]
- Rabindra, N.P.; Subhayan, C.; Pratibha, B.; Janesh, K.; Arindam, G.; Akhilesh, K.S. Seven coordinate Co(II) and six coordinate Ni(II) complexes of an aromatic macrocyclic triamide ligand as paraCEST agents for MRI. Dalton Trans. 2019, 48, 8899–8910. [Google Scholar]
- Rojas-Quijano, F.A.; Benyó, E.B.; Tircsó, G.; Kálmán, F.K.; Baranyai, Z.; Aime, S.; Sherry, D.A.; Kovács, Z. Lanthanide(III) complexes of tris(amide) PCTA derivatives as potential bimodal magnetic resonance and optical imaging agents. Chem. Eur. J. 2009, 15, 13188–13200. [Google Scholar] [CrossRef]
- Marin, C.; Inclán, M.; Ramirez-Macias, I.; Albelda, M.T.; Canas, R.; Clares, M.P.; Gonzalez-Garcia, J.; Rosales, M.J.; Urbanova, K.; Garcia-España, E. In vitro antileishmanial activity of aza-scorpiand macrocycles. Inhibition of the antioxidant enzyme iron superoxide dismutase. Rsc Adv. 2016, 6, 17446–17455. [Google Scholar] [CrossRef]
- Marin, C.; Clares, M.P.; Ramírez-Macías, I.; Blasco, S.; Olmo, F.; Soriano, C.; Verdejo, B.; Rosales, M.J.; Gomez-Herrera, D.; Garcia-España, E.; et al. In vitro activity of scorpiand-like azamacrocycle derivatives in promastigotes and intracellular amastigotes of Leishmania infantum and Leishmania braziliensis. Eur. J. Med. Chem. 2013, 62, 466–477. [Google Scholar] [CrossRef]
- Olmo, F.; Marin, C.; Clares, M.P.; Blasco, S.; Albelda, M.T.; Soriano, C.; Gutiérrez-Sánchez, R.; Arrebola-Vargas, F.; Garcia-España, E.; Sánchez-Moreno, M. Scorpiand-like azamacrocycles prevent the chronic establishment of Trypanosoma cruzi in a murine model. Eur. J. Med. Chem. 2013, 70, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.-H.; Li, C.-Y.; Geng, Z.-R.; Ma, X.-Y.; Wang, Z.-L. A potent antitumor Zn2+ tetraazamacrocycle complex targeting DNA: The fluorescent recognition, interaction and apoptosis studies. Chem. Comm. 2011, 47, 11330–11332. [Google Scholar] [CrossRef] [PubMed]
- Le Fur, M.; Beyler, M.; Molnar, E.; Fougere, O.; Esteban-Gomez, D.; Tircso, G.; Platas-Iglesias, C.; Lepareur, N.; Rousseaux, O.; Tripier, R. Stable and inert Yttrium(III) complexes with pyclen-based ligands bearing pendant picolinate arms: Toward new pharmaceuticals for β-Radiotherapy. Inorg. Chem. 2018, 57, 2051–2063. [Google Scholar] [CrossRef] [PubMed]
- Le Fur, M.; Beyler, M.; Molnar, E.; Fougere, O.; Esteban-Gomez, D.; Tircso, G.; Platas-Iglesias, C.; Lepareur, N.; Rousseaux, O.; Tripier, R. The role of the capping bond effect on pyclen natY3+/90Y3+ chelates: Full control of the regiospecific N-functionalization makes the difference. Chem. Comm. 2017, 53, 9534–9537. [Google Scholar] [CrossRef] [Green Version]
- Guijarro, L.; Inclán, M.; Pitarch-Jarque, J.; Doménech-Carbó, A.; Chicote, J.U.; Trefler, S.; García-España, E.; García-España, A.; Verdejo, B. Homo- and heterobinuclear Cu2+ and Zn2+ complexes of ditopic aza scorpiand ligands as superoxide dismutase mimics. Inorg. Chem. 2017, 56, 13748–13758. [Google Scholar] [CrossRef]
- Serena, C.; Calvo, E.; Clares, M.P.; Diaz, M.L.; Chicote, J.U.; Beltrán-Debon, R.; Fontova, R.; Rodriguez, A.; García-España, E.; García-España, A. Significant in-vivo anti-inflammatory activity of Pytren4Q-Mn a superoxide dismutase 2 (SOD2) mimetic scorpiand-like Mn (II) complex. PLoS ONE 2015, 10, e0119102/1–e0119102/12. [Google Scholar] [CrossRef]
- Lincoln, K.M.; Richardson, T.E.; Rutter, L.; Gonzalez, P.; Simpkins, J.W.; Green, K.N. An N-heterocyclic amine chelate capable of antioxidant capacity and amyloid disaggregation. Acs Chem. Neurosci. 2012, 3, 919–927. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Camarena, Á.; Liberato, A.; Delgado-Pinar, E.G.; Algarra, A.; Pitarch-Jarque, J.; Llinares, J.M.; Mañez, M.Á.; Domenech-Carbó, A.; Basallote, M.G.; García-España, E. Coordination chemistry of Cu2+ complexes of small N-alkylated tetra-azacyclophanes with SOD activity. Inorg. Chem. 2018, 57, 10961–10973. [Google Scholar] [CrossRef]
- Green, K.N.; Pota, K.; Tircsó, G.; Gogolák, R.A.; Kinsinger, O.; Davda, C.; Blain, K.; Brewer, S.M.; Gonzalez, P.; Johnston, H.M.; et al. Dialing in on pharmacological features for therapeutic antioxidant small molecule. Dalton Trans. 2019, 48, 12430–12439. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Ma, X.; Wen, J.; Geng, Z.; Wang, Z. Real-time fluorescence assays of alkaline phosphatase and ATP sulfurylase activities based on a novel PPi fluorescent probe. Talanta 2015, 137, 156–160. [Google Scholar] [CrossRef]
- Wang, X.; Ma, X.; Yang, Z.; Zhang, Z.; Wen, J.; Geng, Z.; Wang, Z. An NBD-armed tetraaza macrocyclic lysosomal-targeted fluorescent probe for imaging copper(II) ions. Chem. Comm. 2013, 49, 11263–11265. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Geng, Z.; Yin, Y.; Wang, Z. A versatile water soluble fluorescent probe for ratiometric sensing of Hg2+ and bovine serum albumin. Dalton Trans. 2011, 40, 9737–9745. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Geng, Z.; Yin, Y.; Zhang, Z.; Wang, Z. A Zn2+-specific turn-on fluorescent probe for ratiometric sensing of pyrophosphate in both water and blood serum. Dalton Trans. 2011, 40, 1984–1989. [Google Scholar] [CrossRef] [PubMed]
- Savastano, M.; Arranz-Mascarós, P.; Clares, M.P.; Cuesta, R.; Godino-Salido, M.L.; Guijarro, L.; Gutiérrez-Valero, M.D.; Inclán, M.; Bianchi, A.; García-España, E.; et al. A new heterogeneous catalyst obtained via supramolecular decoration of graphene with a Pd2+ azamacrocyclic complex. Molecules 2019, 24, 2714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passaponti, M.; Savastano, M.; Clares, M.P.; Inclán, M.; Lavacchi, A.; Bianchi, A.; García-España, E.; Innocenti, M. MWCNTs-supported Pd(II) complexes with high catalytic efficiency in oxygen reduction reaction in alkaline media. Inorg. Chem. 2018, 57, 14484–14488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savastano, M.; Arranz-Mascarós, P.; Bazzicalupi, C.; Clares, M.P.; Godino-Salido, M.L.; Guijarro, L.; Gutiérrez-Valero, M.-D.; Bianchi, A.; García-España, E.; López-Garzón, R. Polyfunctional tetraaza-macrocyclic ligands: Zn(II), Cu(II) binding and formation of hybrid materials with multiwalled carbon nanotubes. ACS Omega 2017, 2, 3868–3877. [Google Scholar] [CrossRef] [PubMed]
- Savastano, M.; Arranz-Mascarós, P.; Bazzicalupi, C.; Clares, M.P.; Godino-Salido, M.L.; Gutiérrez-Valero, M.D.; Inclán, M.; Bianchi, A.; García-España, E.; López-Garzón, R. Construction of green nanostructured heterogeneous catalysts via non-covalent surface decoration of multi-walled carbon nanotubes with Pd(II) complexes of azamacrocycles. J. Catal. 2017, 353, 239–249. [Google Scholar] [CrossRef]
- Serrano-Plana, J.; Aguinaco, A.; Belda, R.; Garcia-España, E.; Basallote, M.G.; Company, A.; Costas, M. Exceedingly fast oxygen atom transfer to olefins via a catalytically competent nonheme iron species. Angew. Chem. Int. Ed. 2016, 55, 6310–6314. [Google Scholar] [CrossRef] [Green Version]
- Wen, J.; Qin, S.; Ma, L.; Dong, L.; Zhang, J.; Liu, S.; Duan, Y.; Chen, S.; Hu, C.; Yu, X. Iron-mediated direct Suzuki-Miyaura reaction: A new method for the ortho-arylation of pyrrole and pyridine. Org. Lett. 2010, 12, 2694–2697. [Google Scholar] [CrossRef]
- Savastano, M.; Martínez-Camarena, Á.; Bazzicalupi, C.; Delgado-Pinar, E.; Llinares, J.M.; Mariani, P.; Verdejo, B.; García-España, E.; Bianchi, A. Stabilization of supramolecular networks of polyiodides with protonated small tetra-azacyclophanes. Inorganics 2019, 7, 48. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, S.E.; Bloom, J.W.G. Anion–p interactions and positive electrostatic potentials of N-heterocycles arise from the positions of the nuclei, not changes in the p-electron distribution. Chem. Commun. 2014, 50, 11118–11121. [Google Scholar] [CrossRef]
- Savastano, M.; García-Gallarín, C.; López de la Torre, M.D.; Bazzicalupi, C.; Bianchi, A.; Melguizo, M. Anion-π and lone pair-π interactions with s-tetrazine-based ligands. Coord. Chem. Rev. 2019, 397, 112–137. [Google Scholar] [CrossRef]
- Savastano, M.; Bazzicalupi, C.; Giorgi, C.; García-Gallarín, C.; López de la Torre, M.D.; Pichierri, F.; Bianchi, A.; Melguizo, M. Anion complexes with tetrazine-based ligands: Formation of strong anion–π interactions in solution and in the solid state. Inorg. Chem. 2016, 55, 8013–8024. [Google Scholar] [CrossRef] [PubMed]
- Savastano, M.; Bazzicalupi, C.; García-Gallarín, C.; Gellini, C.; López de la Torre, M.D.; Mariani, P.; Pichierri, F.; Bianchi, A.; Melguizo, M. Iodide and triiodide anion complexes involving anion–π interactions with a tetrazine-based receptor. Dalton Trans. 2017, 46, 4518–4529. [Google Scholar] [CrossRef] [PubMed]
- Savastano, M.; Bazzicalupi, C.; García-Gallarín, C.; Giorgi, C.; López de la Torre, M.D.; Pichierri, F.; Bianchi, A.; Melguizo, M. Halide and hydroxide anion binding in water. Dalton Trans. 2018, 47, 3329–3338. [Google Scholar] [CrossRef] [PubMed]
- Savastano, M.; García-Gallarín, C.; López de la Torre, M.D.; Pichierri, F.; Bazzicalupi, C.; Bianchi, A.; Melguizo, M. Interplay between salt bridge, hydrogen bond and anion-π interactions in thiocyanate binding. Inorg. Chim. Acta 2018, 470, 133–138. [Google Scholar] [CrossRef]
- Savastano, M.; García-Gallarín, C.; Giorgi, C.; Gratteri, P.; López de la Torre, M.D.; Bazzicalupi, C.; Bianchi, A.; Melguizo, M. Solid state and solution study on the formation of inorganic anion complexes with a series of tetrazine-based ligands. Molecules 2019, 24, 2247. [Google Scholar] [CrossRef] [Green Version]
- Savastano, M.; Bazzicalupi, C.; Mariani, P.; Bianchi, A. Network formation via anion coordination: Crystal structures based on the interplay of non-covalent interactions. Molecules 2018, 23, 572. [Google Scholar] [CrossRef] [Green Version]
- Savastano, M.; Bazzicalupi, C.; García-Gallarín, C.; López de la Torre, M.D.; Bianchi, A.; Melguizo, M. Supramolecular forces and their interplay in stabilizing complexes of organic anions: Tuning binding selectivity in water. Org. Chem. Front. 2019, 6, 75–86. [Google Scholar] [CrossRef]
- Savastano, M.; Bazzicalupi, C.; Gellini, C.; Bianchi, A. Infinite supramolecular pseudo-polyrotaxane with poly[3]catenane axle: Assembling nanosized rings from mono- and diatomic I− and I2 tectons. Chem. Commun. 2020, 56, 551–554. [Google Scholar] [CrossRef]
- Savastano, M.; Bazzicalupi, C.; Gellini, C.; Bianchi, A. Genesis of complex polyiodide networks: Insights on the blue box/I-/I2 ternary system. Crystals 2020, 10, 387. [Google Scholar] [CrossRef]
- Savastano, M.; Bazzicalupi, C.; Bianchi, A. Porous frameworks based on supramolecular ball joints: Bringing flexibility to ordered 3D lattices. Chem. Eur.J. 2020, 26, 5994–6005. [Google Scholar] [CrossRef] [PubMed]
- Spackman, M.A.; Byrom, P.G. A novel definition of a molecule in a crystal. Chem. Phys. Lett. 1997, 267, 215–220. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Spackman, M.A.; Mitchell, A.S. Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Cryst B 2004, 60, 627–668. [Google Scholar] [CrossRef] [PubMed]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Arranz, P.; Bianchi, A.; Cuesta, R.; Giorgi, C.; Godino, M.L.; Gutiérrez, M.D.; López, R.; Santiago, A. Binding and removal of sulfate, phosphate, arsenate, tetrachloromercurate, and chromate in aqueous solution by means of an activated carbon functionalized with a pyrimidine-based anion receptor (HL). Crystal structures of [H3L(HgCl4)]·H2O and [H3L(HgBr4)]·H2O showing anion−π interactions. Inorg. Chem. 2010, 49, 9321–9332. [Google Scholar]
- Arranz-Mascarós, P.; Bazzicalupi, C.; Bianchi, A.; Giorgi, C.; Godino-Salido, M.-L.; Gutiérrez-Valero, M.-D.; Lopez-Garzón, R.; Savastano, M. Thermodynamics of Anion−π interactions in aqueous solution. J. Am. Chem. Soc. 2013, 135, 102–105. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Arca, M.; Devillanova, F.A.; Hursthouse, M.B.; Huth, S.L.; Isaia, F.; Lippolis, V.; Mancini, A.; Verani, G. Reactions of halogens/interhalogens with polypyridyl substrates: The case of 2,4,6-Tris(2-Pyridyl)-1,3,5-Triazine. Eur. J. Inorg. Chem. 2008, 3921–3928. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl.Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17; University of Western Australia: Crawley, Australia, 2017. [Google Scholar]
- Inkscape. Available online: https://inkscape.org (accessed on 25 May 2020).
Sample Availability: Samples of the compound are not available from the authors. |














| D⋅⋅⋅A | Distance (Å) | H⋅⋅⋅A | Distance (Å) | D-H⋅⋅⋅A | Angle (deg) |
|---|---|---|---|---|---|
| N3⋅⋅⋅O1 | 2.94(1) | H3B⋅⋅⋅O1 | 2.429 | N3-H3B⋅⋅⋅O1 | 116.0 |
| N1⋅⋅⋅O2 | 2.92(1) | H1A⋅⋅⋅O2 | 2.359 | N1-H1A⋅⋅⋅O2 | 119.4 |
| N3⋅⋅⋅O2′ (−x,−y,−z) | 2.931(9) | H3B⋅⋅⋅O2′ | 2.317 | N3-H3B⋅⋅⋅O2′ | 124.5 |
| N1⋅⋅⋅O1′ (−x,−y,−z) | 2.96(1) | H1A⋅⋅⋅O1′ | 2.389 | N1-H1A⋅⋅⋅O1′ | 120.8 |
| N1⋅⋅⋅O34 | 2.828(9) | H1B⋅⋅⋅O34 | 2.041 | N1-H1B⋅⋅⋅O34 | 143.9 |
| N3⋅⋅⋅O34′(−x,−y,−z) | 2.83(1) | H3A⋅⋅⋅O34′ | 2.055 | N3-H3A⋅⋅⋅O34′ | 142.4 |
| N3⋅⋅⋅O32′(−x,−y,−z) | 2.82(2) | H3A⋅⋅⋅O32′ | 2.140 | N3-H3A⋅⋅⋅O32′ | 130.5 |
| Br2′⋅⋅⋅C22 (−x + 1,−y + 1,−z) | 3.90(1) | Br2′⋅⋅⋅H22 | 2.987 | C22-H22⋅⋅⋅Br2′ | 162.2 |
| Br2⋅⋅⋅C26 (−x,−y + 1,−z) | 3.79(1) | Br2⋅⋅⋅H26 | 2.904 | C26-H26⋅⋅⋅Br2 | 154.7 |
| Br4⋅⋅⋅C17 (−x,−y + 1,−z) | 3.73(1) | Br4⋅⋅⋅H17A | 3.026 | C17-H17A⋅⋅⋅Br4 | 128.8 |
| Br4′⋅⋅⋅C19 (−x + 1,−y + 1,−z) | 3.71(1) | Br4′⋅⋅⋅H19B | 2.986 | C19-H19B⋅⋅⋅Br4′ | 131.3 |
| Br5⋅⋅⋅C16 | 3.89(1) | Br5⋅⋅⋅H16B | 3.004 | C16-H16B⋅⋅⋅Br5 | 150.2 |
| Br5⋅⋅⋅C13 | 3.680(7) | Br5⋅⋅⋅H13A | 2.812 | C13-H13A⋅⋅⋅Br5 | 146.7 |
| Br5⋅⋅⋅C25 (−x,−y + 1,−z) | 3.89(2) | Br5⋅⋅⋅H25 | 2.947 | C25-H25⋅⋅⋅Br5 | 173.3 |
| Empirical Formula | C18H26Br4.5N4.5O3.5S |
|---|---|
| Formula weight | 753.09 |
| Temperature (K) | 100 |
| Crystal system | triclinic |
| Space group | P-1 |
| a (Å) | 9.8426(9) |
| b (Å) | 11.428(1) |
| c (Å) | 13.413(1) |
| α (°) | 105.072(3) |
| β (°) | 99.065(3) |
| γ (°) | 115.435(3) |
| Volume (Å3) | 1251.3(2) |
| Z | 2 |
| Independent reflections/R(int) | 4423/0.0625 |
| μ (mm−1) | 9.838 (Cu-Kα) |
| R indices [I > 2σ(I)] a | R1 = 0.1045 |
| wR2 = 0.2905 | |
| R indices (all data) a | R1 = 0.1083 |
| wR2 = 0.2943 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Camarena, Á.; Savastano, M.; Bazzicalupi, C.; Bianchi, A.; García-España, E. Stabilisation of Exotic Tribromide (Br3−) Anions via Supramolecular Interaction with a Tosylated Macrocyclic Pyridinophane. A Serendipitous Case. Molecules 2020, 25, 3155. https://doi.org/10.3390/molecules25143155
Martínez-Camarena Á, Savastano M, Bazzicalupi C, Bianchi A, García-España E. Stabilisation of Exotic Tribromide (Br3−) Anions via Supramolecular Interaction with a Tosylated Macrocyclic Pyridinophane. A Serendipitous Case. Molecules. 2020; 25(14):3155. https://doi.org/10.3390/molecules25143155
Chicago/Turabian StyleMartínez-Camarena, Álvaro, Matteo Savastano, Carla Bazzicalupi, Antonio Bianchi, and Enrique García-España. 2020. "Stabilisation of Exotic Tribromide (Br3−) Anions via Supramolecular Interaction with a Tosylated Macrocyclic Pyridinophane. A Serendipitous Case" Molecules 25, no. 14: 3155. https://doi.org/10.3390/molecules25143155
APA StyleMartínez-Camarena, Á., Savastano, M., Bazzicalupi, C., Bianchi, A., & García-España, E. (2020). Stabilisation of Exotic Tribromide (Br3−) Anions via Supramolecular Interaction with a Tosylated Macrocyclic Pyridinophane. A Serendipitous Case. Molecules, 25(14), 3155. https://doi.org/10.3390/molecules25143155