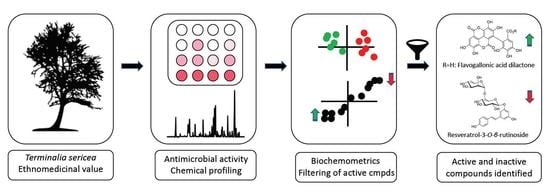
Unravelling the Antibacterial Activity of Terminalia sericea Root Bark through a Metabolomic Approach
Abstract
:1. Introduction
2. Results and Discussion
2.1. Untargeted UPLC-MS and Discriminant Analysis
2.2. Isolation and Identification of Chemical Constituents
2.3. Antibacterial Constituents of T. sericea Root Bark
2.4. Biochemometric Analysis
3. Materials and Methods
3.1. Sampling and Sample Preparation
3.2. UPLC-MS Analysis
3.3. Chemometric Analysis of LC-MS Data
3.4. Isolation of Chemical Markers
3.5. Purification Using Preparative-HPLC-MS
3.6. Nuclear Magnetic Resonance (NMR) Spectroscopy
3.7. Evaluation of the Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moshi, M.J.; Mbwambo, Z.H. Some pharmacological properties of extracts of Terminalia sericea roots. J. Ethnopharmacol. 2005, 97, 43–47. [Google Scholar] [CrossRef]
- Likoswe, M.G.; Njoloma, J.P.; Mwase, W.F.; Chilima, C.Z. Effect of seed collection times and pretreatment methods on germination of Terminalia sericea Burch. ex DC. Afr. J. Biotechnol. 2008, 7, 2840–2846. [Google Scholar]
- Chivandi, E.; Davidson, B.; Erlwanger, K. Proximate, mineral, fibre, phytate–phosphate, vitamin E, amino acid and fatty acid composition of Terminalia sericea. S. Afr. J. Bot. 2013, 88, 96–100. [Google Scholar] [CrossRef] [Green Version]
- van Wyk, B.-E.; van Oudtshoorn, B.; Gericke, N. Medicinal Plants of South Africa, 2nd ed.; Briza Publications: Pretoria, South Africa, 2013; p. 288. [Google Scholar]
- Rukangira, E. Medicinal plants and traditional medicine in Africa: Constraints and challenges. Sustain. Dev. Int. 2001, 4, 179–184. [Google Scholar]
- Rode, T. Complex formation of sericoside with hydrophilic cyclodextrins: Improvement of solubility and skin penetration in topical emulsion based formulations. Eur. J. Pharm. Biopharm. 2003, 55, 191–198. [Google Scholar] [CrossRef]
- Pagin, I.; Togni, S.; Maramaldi, G.; Cattaneo, R.; Caccia, G.; Eggenhoffner, R.; Giacomelli, L. Anti-aging effects of a novel sericoside 0.5% cream in reducing skin wrinkles and ameliorating skin texture. Dermatol. Exp. 2016, 18, 183–186. [Google Scholar]
- Katerere, D.R.; Gray, A.I.; Nash, R.J.; Waigh, R.D. Phytochemical and antimicrobial investigations of stilbenoids and flavonoids isolated from three species of Combretaceae. Fitoterapia 2012, 83, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Eloff, J.N. The antibacterial activity of 27 southern African members of the Combretaceae. S. Afr. J. Sci. 1999, 95, 148–152. [Google Scholar]
- Fyhrquist, P.; Mwasumbi, L.; Hæggström, C.-A.; Vuorela, H.; Hiltunen, R.; Vuorela, P. Ethnobotanical and antimicrobial investigation on some species of Terminalia and Combretum (Combretaceae) growing in Tanzania. J. Ethnopharmacol. 2002, 79, 169–177. [Google Scholar] [CrossRef]
- Steenkamp, V.; Mathivha, E.; Gouws, M.; Van Rensburg, C. Studies on antibacterial, antioxidant and fibroblast growth stimulation of wound healing remedies from South Africa. J. Ethnopharmacol. 2004, 95, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Eldeen, I.M.; Elgorashi, E.E.; van Staden, J. Antibacterial, anti-inflammatory, anti-cholinesterase and mutagenic effects of extracts obtained from some trees used in South African traditional medicine. J. Ethnopharmacol. 2005, 102, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Tshikalange, T.; Meyer, J.; Hussein, A. Antimicrobial activity, toxicity and the isolation of a bioactive compound from plants used to treat sexually transmitted diseases. J. Ethnopharmacol. 2005, 96, 515–519. [Google Scholar] [CrossRef] [PubMed]
- York, T.; van Vuuren, S.F.; de Wet, H. An antimicrobial evaluation of plants used for the treatment of respiratory infections in rural Maputaland, KwaZulu-Natal, South Africa. J. Ethnopharmacol. 2012, 144, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Mabona, U.; Van Vuuren, S.F. Southern African medicinal plants used to treat skin diseases. S. Afr. J. Bot. 2013, 87, 175–193. [Google Scholar] [CrossRef] [Green Version]
- Cock, I.E.; van Vuuren, S.F. Anti-Proteus activity of some South African medicinal plants: Their potential for the prevention of rheumatoid arthritis. Inflammopharmacology 2014, 22, 23–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cock, I.E.; van Vuuren, S.F. The potential of selected South African plants with anti-Klebsiella activity for the treatment and prevention of ankylosing spondylitis. Inflammopharmacology 2015, 23, 21–35. [Google Scholar] [CrossRef] [Green Version]
- van Vuuren, S.F.; Nkwanyana, M.N.; de Wet, H. Antimicrobial evaluation of plants used for the treatment of diarrhoea in a rural community in northern Maputaland, KwaZulu-Natal, South Africa. BMC Complement. Altern. Med. 2015, 15, 53. [Google Scholar] [CrossRef] [Green Version]
- Netshiluvhi, T.; Eloff, J. Influence of annual rainfall on antibacterial activity of acetone leaf extracts of selected medicinal trees. S. Afr. J. Bot. 2016, 102, 197–201. [Google Scholar] [CrossRef]
- Eldeen, I.M.; Elgorashi, E.E.; Mulholland, D.A.; van Staden, J. Anolignan B: A bioactive compound from the roots of Terminalia sericea. J. Ethnopharmacol. 2006, 103, 135–138. [Google Scholar] [CrossRef]
- Eldeen, I.M.; Van Heerden, F.R.; Van Staden, J. Isolation and biological activities of termilignan B and arjunic acid from Terminalia sericea roots. Planta Med. 2008, 74, 411–413. [Google Scholar] [CrossRef]
- Ulrich-Merzenich, G.S. Combination screening of synthetic drugs and plant derived natural products—Potential and challenges for drug development. Synergy 2014, 1, 59–69. [Google Scholar] [CrossRef]
- Faruque, M.O.; Ankhi, U.R.; Kamaruzzaman, M.; Barlow, J.W.; Zhou, B.; Hao, J.; Yang, X.; Hu, X. Chemical composition and antimicrobial activity of Congea tomentosa, an ethnomedicinal plant from Bangladesh. J. Ind. Crop. Prod. 2019, 141, 111745. [Google Scholar] [CrossRef]
- Abdelgaleil, S.; Saad, M.; Ariefta, N.; Shiono, Y. Antimicrobial and phytotoxic activities of secondary metabolites from Haplophyllum tuberculatum and Chrysanthemum coronarium. S. Afr. J. Bot. 2020, 128, 35–41. [Google Scholar] [CrossRef]
- Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. J. Phytochem. 2003, 64, 3–19. [Google Scholar] [CrossRef]
- Bombardelli, E.; Bonati, A.; Gabetta, B.; Mustich, G. Triterpenoids of Terminalia sericea. Phytochemistry 1974, 13, 2559–2562. [Google Scholar] [CrossRef]
- Bombardelli, E.; Martinelli, E.; Mustich, G. Plants of Mozambique. IX. A new hydroxystilbene glycoside from Terminalia sericea. Fitoterapia 1975, 46, 199–200. [Google Scholar]
- Joseph, C.C.; Moshi, M.; Innocent, E.; Nkunya, M. Isolation of a stilbene glycoside and other constituents of Terminalia sericeae. Afr. J. Tradit. Complement. Altern. Med. 2007, 4, 383–386. [Google Scholar] [CrossRef] [Green Version]
- Hess, S.C.; Monache, F.D. Divergioic acid, a triterpene from Vochysia divergens. J. Braz. Chem. Soc. 1999, 10, 104–106. [Google Scholar] [CrossRef] [Green Version]
- Rahman, A.-u.; Zareen, S.; Choudhary, M.I.; Akhtar, M.N.; Ngounou, F. Some chemical constituents of Terminalia glaucescens and their enzymes inhibition activity. Z. Naturforsch. C J. Biosci. 2005, 60, 347–350. [Google Scholar] [CrossRef]
- Tchuenmogne, T.; Aimée, M.; Kammalac, T.N.; Gohlke, S.; Kouipou, R.M.T.; Aslan, A.; Kuzu, M.; Comakli, V.; Demirdag, R.; Ngouela, S.A. Compounds from Terminalia mantaly L.(Combretaceae) stem bark exhibit potent inhibition against some pathogenic yeasts and enzymes of metabolic significance. Medicines 2017, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- Asres, K.; Bucar, F.; Edelsbrunner, S.; Kartnig, T.; Höger, G.; Thiel, W. Investigations on antimycobacterial activity of some Ethiopian medicinal plants. Phytother. Res. 2001, 15, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Gossan, D.P.A.; Magid, A.A.; Yao-Kouassi, P.A.; Josse, J.; Gangloff, S.C.; Morjani, H.; Voutquenne-Nazabadioko, L. Antibacterial and cytotoxic triterpenoids from the roots of Combretum racemosum. Fitoterapia 2016, 110, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Wanjala, C.C.; Majinda, R.R. A new stilbene glycoside from Elephantorrhiza goetzei. Fitoterapia 2001, 72, 649–655. [Google Scholar] [CrossRef]
- Kim, J.-P.; Lee, I.-K.; Yun, B.-S.; Chung, S.-H.; Shim, G.-S.; Koshino, H.; Yoo, I.-D. Ellagic acid rhamnosides from the stem bark of Eucalyptus globulus. Phytochemistry 2001, 57, 587–591. [Google Scholar] [CrossRef]
- Srivastava, A.; Jagan Mohan Rao, L.; Shivanandappa, T. Isolation of ellagic acid from the aqueous extract of the roots of Decalepis hamiltonii: Antioxidant activity and cytoprotective effect. Food Chem. 2007, 103, 224–233. [Google Scholar] [CrossRef]
- Orabi, M.A.; Yoshimura, M.; Amakura, Y.; Hatano, T. Ellagitannins, gallotannins, and gallo-ellagitannins from the galls of Tamarix aphylla. Fitoterapia 2015, 104, 55–63. [Google Scholar] [CrossRef]
- Mohieldin, E.A.M.; Muddathir, A.M.; Yamauchi, K.; Mitsunaga, T. Anti-caries activity of selected Sudanese medicinal plants with emphasis on Terminalia laxiflora. Rev. Bras. Farmacogn. 2017, 27, 611–618. [Google Scholar] [CrossRef]
- Salih, E.Y.; Fyhrquist, P.; Abdalla, A.; Abdelgadir, A.Y.; Kanninen, M.; Sipi, M.; Luukkanen, O.; Fahmi, M.K.; Elamin, M.H.; Ali, H.A.J.A. LC-MS/MS tandem mass spectrometry for analysis of phenolic compounds and pentacyclic triterpenes in antifungal extracts of Terminalia brownii (Fresen). Antibiotics 2017, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- Marzouk, M.S.; El-Toumy, S.A.; Moharram, F.A. Pharmacologically active ellagitannins from Terminalia myriocarpa. Planta Med. 2002, 68, 523–527. [Google Scholar] [CrossRef]
- Okasaka, M.; Takaishi, Y.; Kogure, K.; Fukuzawa, K.; Shibata, H.; Higuti, T.; Honda, G.; Ito, M.; Kodzhimatov, O.K.; Ashurmetov, O. New stilbene derivatives from Calligonum leucocladum. J. Nat. Prod. 2004, 67, 1044–1046. [Google Scholar] [CrossRef]
- Estrada, O.; Hasegawa, M.; Gonzalez-Mujica, F.; Motta, N.; Perdomo, E.; Solorzano, A.; Mendez, J.; Mendez, B.; Zea, E.G. Evaluation of flavonoids from Bauhinia megalandra leaves as inhibitors of glucose-6-phosphatase system. Phytother. Res. 2005, 19, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Bentley, J.; Moore, J.P.; Farrant, J.M. Metabolomic profiling of the desiccation-tolerant medicinal shrub Myrothamnus flabellifolia indicates phenolic variability across its natural habitat: Implications for tea and cosmetics production. Molecules 2019, 24, 1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, D.; Gupta, M.; Tripathi, A.; Prajapati, V.; Kumar, S. Arjunetin from Terminalia arjuna as an insect feeding-deterrent and growth inhibitor. Phytother. Res. 2004, 18, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Salih, E.Y.A.; Kanninen, M.; Sipi, M.; Luukkanen, O.; Hiltunen, R.; Vuorela, H.; Julkunen-Tiitto, R.; Fyhrquist, P. Tannins, flavonoids and stilbenes in extracts of African savanna woodland trees Terminalia brownii, Terminalia laxiflora and Anogeissus leiocarpus showing promising antibacterial potential. S. Afr. J. Bot. 2017, 108, 370–386. [Google Scholar] [CrossRef]
- Eloff, J. Which extractant should be used for the screening and isolation of antimicrobial components from plants? J. Ethnopharmacol. 1998, 60, 1–8. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds may be available from the authors. |





| Samples | B.c | S.a | S.e | E.c | K.p | P.a | S.s | S.t |
|---|---|---|---|---|---|---|---|---|
| CE | 0.29 | 1.50 | 1.50 | 1.50 | 3.00 | 0.75 | 1.50 | 1.50 |
| F1 F2 | 0.29 >3.00 | 0.75 3.00 | 0.75 1.50 | 1.50 >3.0 | 1.50 3.00 | 0.75 1.50 | 1.50 1.50 | 1.50 1.50 |
| (1) | 0.38 | 0.75 | 0.75 | 0.38 | 0.38 | 0.38 | 0.29 | >0.75 |
| (2) | >1.30 | >1.30 | >1.30 | >1.30 | >1.30 | >1.30 | >1.30 | >1.30 |
| (3) | >1.30 | >1.30 | >1.30 | >1.30 | 1.30 | >1.30 | >1.30 | >1.30 |
| (4) | 0.22 | 0.75 | 0.75 | 0.38 | >0.75 | 0.38 | 0.57 | 0.38 |
| (5) | 0.11 | 0.50 | 0.75 | 0.38 | 0.38 | 0.38 | 0.75 | 0.25 |
| (6) | 0.12 | 0.75 | 0.75 | 0.38 | 0.38 | 0.38 | 0.38 | 0.32 |
| (7) | 0.05 | NT | 0.75 | 0.38 | >0.75 | NT | 0.38 | 0.38 |
| (9) | 0.14 | NT | >0.75 | 0.80 | >0.75 | NT | 0.38 | 0.75 |
| Cipro | 0.04 | 0.08 | 0.08 | 1.25 | 0.08 | 0.08 | 1.25 | 0.04 |
| Population | B. cereus | S. typhi |
|---|---|---|
| P1.1 | 1.0 | 0.50 |
| P1.2 | 1.0 | 0.25 |
| P1.3 | 1.0 | 0.25 |
| P2.1 | 1.0 | 0.25 |
| P2.2 | 1.0 | 0.25 |
| P2.3 | 1.0 | 0.50 |
| P2.4 | 1.0 | 0.50 |
| P3.1 | 1.0 | 0.25 |
| P3.2 | 1.0 | 0.25 |
| P3.3 | 1.0 | 0.25 |
| P3.4 | 1.0 | 0.25 |
| P4.1 | 1.0 | 1.0 |
| P4.2 | 1.0 | 1.0 |
| P4.3 | 1.0 | 1.0 |
| P4.4 | 1.0 | 1.0 |
| P4.5 | 1.0 | 1.0 |
| P5.1 | 1.0 | 1.0 |
| P5.2 | 1.0 | 1.0 |
| P5.3 | 1.0 | 1.0 |
| P5.4 | 1.0 | 1.0 |
| P6.1 | 1.0 | 1.0 |
| P6.2 | 1.0 | 1.0 |
| P6.3 | 1.0 | 1.0 |
| P6.4 | 1.0 | 1.0 |
| P6.5 | 1.0 | 1.0 |
| P7.1 | 1.0 | 1.0 |
| P7.2 | 0.75 | 0.50 |
| P7.3 | 1.0 | 0.50 |
| P7.4 | 1.0 | 0.50 |
| P7.5 | 1.0 | 0.50 |
| P8.1 | 1.0 | 0.50 |
| P8.2 | 1.0 | 0.50 |
| P8.3 | 1.0 | 0.50 |
| P8.4 | 1.0 | 0.50 |
| P8.5 | 1.0 | 0.50 |
| P9.1 | 1.0 | 0.50 |
| P9.2 | 1.0 | 0.50 |
| P9.3 | 1.0 | 0.50 |
| P9.4 | 1.0 | 0.50 |
| P9.5 | 1.0 | 0.25 |
| P10.1 | 1.0 | 0.50 |
| P10.2 Cipro * | 1.0 0.04 | 0.50 0.04 |
| Popu-lation | Code | Location | District | Coordinates | No of Samples | Voucher No |
|---|---|---|---|---|---|---|
| BP | Bela-Bela/ Pretoria (N1) | Waterberg | S24°47′51.9″ E28°27′03.6″ | 3 | CPA001 | |
| P2 | G | Muyexe, Giyani | Mokopani | S23°11′22.6″ E30°55′05.3″ | 4 | CPA002 |
| J | Maila, close to the N1 | Vhembe | S23°14′47.0″ E29°53′06.8″ | 4 | CPA003 | |
| P4 | K | Along Punda Maria/Kruger road | Vhembe | S22°58′22.0″ E30°27′27.0″ | 5 | CPA004 |
| P5 | MM | Mavambe, Malamulele | Vhembe | S23°00′02.1″ E30°39′09.0″ | 4 | CPA005 |
| P6 | MP | Mokopong/Pretoria along N1 | Waterberg | S24°20′41.6″ E28°53′47.4″ | 5 | CPA006 |
| P7 | TSA | Tshandama, Thengwe | Vhembe | S22°45′43.0″ E30°30′34.3″ | 5 | CPA007 |
| P8 | TSH | Tshitavha, Sambandou | Vhembe | S22°44′41.2″ E30°38′41.2″ | 5 | CPA008 |
| P9 | TZ | Modjadjiskloof, along Tzaneen road | Mokopani | S23°33′14.4″ E30°03′55.6″ | 5 | CPA009 |
| P10 | V | Vuwani | Vhembe | S23°07′46.8″ E30°22′46.9″ | 2 | CPA010 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anokwuru, C.P.; Tankeu, S.; van Vuuren, S.; Viljoen, A.; Ramaite, I.D.I.; Taglialatela-Scafati, O.; Combrinck, S. Unravelling the Antibacterial Activity of Terminalia sericea Root Bark through a Metabolomic Approach. Molecules 2020, 25, 3683. https://doi.org/10.3390/molecules25163683
Anokwuru CP, Tankeu S, van Vuuren S, Viljoen A, Ramaite IDI, Taglialatela-Scafati O, Combrinck S. Unravelling the Antibacterial Activity of Terminalia sericea Root Bark through a Metabolomic Approach. Molecules. 2020; 25(16):3683. https://doi.org/10.3390/molecules25163683
Chicago/Turabian StyleAnokwuru, Chinedu P, Sidonie Tankeu, Sandy van Vuuren, Alvaro Viljoen, Isaiah D. I Ramaite, Orazio Taglialatela-Scafati, and Sandra Combrinck. 2020. "Unravelling the Antibacterial Activity of Terminalia sericea Root Bark through a Metabolomic Approach" Molecules 25, no. 16: 3683. https://doi.org/10.3390/molecules25163683
APA StyleAnokwuru, C. P., Tankeu, S., van Vuuren, S., Viljoen, A., Ramaite, I. D. I., Taglialatela-Scafati, O., & Combrinck, S. (2020). Unravelling the Antibacterial Activity of Terminalia sericea Root Bark through a Metabolomic Approach. Molecules, 25(16), 3683. https://doi.org/10.3390/molecules25163683