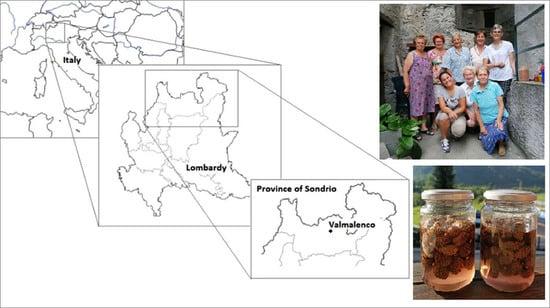
Using Medicinal Plants in Valmalenco (Italian Alps): From Tradition to Scientific Approaches
Abstract
:1. Introduction
2. Results and Discussion
2.1. Field Work
2.2. Bibliographic Research
2.3. Pinus mugo Turra
3. Materials and Methods
- Preliminary Investigation on the Territory and the Local Flora
- Interview Management and Supporting Material
- Collection of Plant Material, Production of Herbarium Samples, and Photographic Archive
- Data Archiving and Analysis
Scientific Confirmation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bussmann, R.W. Ethnobotany of Mountain Regions: Far Eastern Europe. In Ethnobotany of the Mountain Regions of Far Eastern Europe. Ethnobotany of Mountain Regions; Batsatsashvili, K., Kikvidze, Z., Bussmann, R.W., Eds.; Springer International Publishing: Berlin, Germany, 2019. [Google Scholar]
- Bellia, G.; Pieroni, A. Isolated, but Transnational: The Glocal Nature of Waldensian Ethnobotany, Western Alps, NW Italy. J. Ethnobiol. Ethnomed. 2015, 11, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dei Cas, L.; Pugni, F.; Fico, G. Tradition of use on medicinal species in Valfurva (Sondrio, Italy). J. Ethnopharmacol. 2015, 163, 113–134. [Google Scholar] [CrossRef] [PubMed]
- Pilgrim, S.E.; Cullen, L.C.; Smith, D.J.; Pretty, J. Ecological knowledge is lost in wealthier communities and countries? Environ. Sci. Technol. 2008, 42, 1002–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva, N.A.; Alves, Â.G.; Chaves, A.; de Ulysses, P.; Ramos, M.A. A biocultural approach to the use of natural resources in Northeast Brazil: A socioeconomic perspective. Acta Bot. Brasilica 2019, 33, 315–330. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, U.P. Re-examining hypotheses concerning the use and knowledge of medicinal plants: A study in the Caatinga vegetation of NE Brazil. J. Ethnobiol. Ethnomed. 2006, 2, 30. [Google Scholar] [CrossRef] [Green Version]
- Dutfield, G. Why traditional knowledge is important in drug discovery. Future Med. Chem. 2010, 2, 1405–1409. [Google Scholar] [CrossRef]
- Süntar, I. Importance of ethnopharmacological studies in drug discovery: Role of medicinal plants. Phytochem. Rev. 2019. [Google Scholar] [CrossRef]
- Andrade-Cetto, A.; Heinrich, M. From the field into the lab: Useful approaches to selecting species based on local knowledge. Front. Pharmacol. 2011, 2, 20. [Google Scholar] [CrossRef] [Green Version]
- Ferranti, R. Flora Alpina di Valtellina e Valchiavenna; LYASIS EDIZIONI: Sondrio, Italy, 2005. [Google Scholar]
- Staub, P.O.; Geck, M.S.; Weckerle, C.S.; Casu, L.; Leonti, M. Classifying diseases and remedies in ethnomedicine and ethnopharmacology. J. Ethnopharmacol. 2015, 174, 514–519. [Google Scholar] [CrossRef] [Green Version]
- Cook, F.E. Economic Botanic Data Collection Standard; Prendergast, H., Ed.; Royal Botanic Gardens: Lodon, UK, 1995. [Google Scholar]
- Vitalini, S.; Iriti, M.; Puricelli, C.; Ciuchi, D.; Segale, A.; Fico, G. Traditional knowledge on medicinal and food plants used in Val San Giacomo (Sondrio, Italy)—An alpine ethnobotanical study. J. Ethnopharmacol. 2013, 145, 517–529. [Google Scholar] [CrossRef]
- Cornara, L.; La Rocca, A.; Terrizzano, L.; Dente, F.; Mariotti, M.G. Ethnobotanical and phytomedical knowledge in the North-Western Ligurian Alps. J. Ethnopharmacol. 2014, 155, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Vitalini, S.; Puricelli, C.; Mikerezi, I.; Iriti, M. Plants, people and traditions: Ethnobotanical survey in the Lombard Stelvio National Park and neighbouring areas (Central Alps, Italy). J. Ethnopharmacol. 2015, 173, 435–458. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Ankli, A.; Frei, B.; Weimann, C.; Sticher, O. Medicinal plants in Mexico: Healers’ consensus and cultural importance. Soc. Sci. Med. 1998, 47, 1859–1871. [Google Scholar] [CrossRef]
- Bruschi, P.; Sugni, M.; Moretti, A.; Signorini, M.A.; Fico, G. Children’s versus adult’s knowledge of medicinal plants: An ethnobotanical study in Tremezzina (Como, Lombardy, Italy). Braz. J. Pharmacogn. 2019, 29, 644–655. [Google Scholar] [CrossRef]
- Pieroni, A.; Giusti, M.E. Alpine ethnobotany in Italy: Traditional knowledge of gastronomic and medicinal plants among the Occitans of the upper Varaita valley, Piedmont. J. Ethnobiol. Ethnomed. 2009, 5, 32. [Google Scholar] [CrossRef] [Green Version]
- Vitalini, S.; Tomè, F.; Fico, G. Traditional uses of medicinal plants in Valvestino (Italy). J. Ethnopharmacol. 2009, 121, 106–116. [Google Scholar] [CrossRef]
- Rivera, D.; Alcaraz, F.; Obón, C. Wild and cultivated plants used as food and medicine by the Cimbrian ethnic minority in the Alps. I Int. Symp. Med. Aromat. Nutraceutical Plants Mt. Areas 2011, 955, 31–39. [Google Scholar] [CrossRef]
- Obón, C.; Rivera, D.; Alcaraz, F. Wild and cultivated plants used as food and medicine by the mòcheni ethnic minority in the Alps. Acta Hortic. 2012, 955, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Mattalia, G.; Quave, C.L.; Pieroni, A. Traditional uses of wild food and medicinal plants among Brigasc, Kyé, and Provençal communities on the Western Italian Alps. Genet.Resour.Crop. Evol. 2013, 60, 587–603. [Google Scholar] [CrossRef]
- WHO. WHO Monographs on Selected Medicinal Plants; WHO Library Cataloguing in Publication Data: Geneva, Switzerland, 2002; Volume 2. [Google Scholar]
- Ali, S.I.; Gopalakrishnan, B.; Venkatesalu, V. Pharmacognosy, Phytochemistry and Pharmacological Properties of Achillea millefolium L.: A Review. Phyther. Res. 2017, 31, 1140–1161. [Google Scholar] [CrossRef]
- Applequist, W.L.; Moerman, D.E. Yarrow (Achillea millefolium L.): A Neglected Panacea? A Review of Ethnobotany, Bioactivity, and Biomedical Research1. Econ. Bot. 2011, 65, 209–225. [Google Scholar] [CrossRef]
- Vitalini, S.; Madeo, M.; Tava, A.; Iriti, M.; Vallone, L.; Avato, P.; Cocuzza, C.; Simonetti, P.; Argentieri, M. Chemical Profile, Antioxidant and Antibacterial Activities of Achillea moschata Wulfen, an Endemic Species from the Alps. Molecules 2016, 21, 830. [Google Scholar] [CrossRef] [Green Version]
- D’Agostino, M.; Dini, I.; Ramundo, E.; Senatore, F. Flavonoid glycosides of Alchemilla vulgaris L. Proc. Phytother. Res. 1998, 12, S162–S163. [Google Scholar] [CrossRef]
- Boroja, T.; Mihailović, V.; Katanić, J.; Pan, S.-P.; Nikles, S.; Imbimbo, P.; Monti, D.M.; Stanković, N.; Stanković, M.S.; Bauer, R. The biological activities of roots and aerial parts of Alchemilla vulgaris L. S. Afr. J. Bot. 2018, 116, 175–184. [Google Scholar] [CrossRef]
- WHO. WHO Monographs on Selected Medicinal Plants; WHO Library Cataloguing in Publication Data: Geneva, Switzerland, 2007; Volume 3. [Google Scholar]
- Kriplani, P.; Guarve, K.; Baghael, U.S. Arnica montana L.—A plant of healing: Review. J. Pharm. Pharmacol. 2017, 69, 925–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capasso, F.; Grandolini, G.; Izzo, A.A. Fitoterapia. Impiego Razionale delle Droghe Vegetali.; Springer: Berlin, Germany, 2006. [Google Scholar]
- Sharma, S.; Arif, M.; Nirala, R.K.; Gupta, R.; Thakur, S.C. Cumulative therapeutic effects of phytochemicals in Arnica montana flower extract alleviated collagen-induced arthritis: Inhibition of both pro-inflammatory mediators and oxidative stress. J. Sci. Food Agric. 2015, 96, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Hall, I.H.; Starenes, C.O.; Lee, K.H.; Waddell, T.G. Mode of action of sesquiterpene lactones as anti-inflammatory agents. J. Pharm. Sci. 1980, 69, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Hall, I.H.; Lee, K.H.; Starenes, C.O.; Sumida, Y.; Wu, R.Y.; Waddell, T.G.; Cochran, J.W.; Gerhart, K.G. Anti-inflammatory activity of sesquiterpene lactones and related compounds. J. Pharm. Sci. 1979, 68, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, S.L.; Belcher, H.J. Use of Arnica to relieve pain after carpal-tunnel release surgery. Altern.Ther.Health Med. 2002, 8, 66–68. [Google Scholar]
- Lyss, G.; Schmidt, T.J.; Merfort, I.; Pahl, H.L. Helenalin, an anti-inflammatory sesquiterpene lactone from Arnica, selectively inhibits transcription factor NF-κB. Biol. Chem. 1997, 378, 951–961. [Google Scholar] [CrossRef]
- McMullen, M.K.; Whitehouse, J.M.; Whitton, P.A.; Towell, A. Bitter tastants alter gastric-phase postprandial haemodynamics. J. Ethnopharmacol. 2014, 154, 719–727. [Google Scholar] [CrossRef] [PubMed]
- McMullen, M.K.; Whitehouse, J.M.; Towell, A. Bitters: Time for a new paradigm. Evid. Based Complement. Altern. Med. 2015, 2015, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brockhoff, A.; Behrens, M.; Massarotti, A.; Appending, G.; Meyerhof, W. Broad tuning of the human bitter taste receptor hTAS2R46 to various sesquiterpene lactones, clerodane and labdane diterpenoids, strychnine, and denatonium. J. Agric. Food Chem. 2007, 55, 6236–6243. [Google Scholar] [CrossRef] [PubMed]
- Tafti, L.D.; Shariatpanahi, S.M.; Mahdavi Damghani, M.; Javadi, B. Traditional Persian topical medications for gastrointestinal diseases. Iran. J. Basic Med. Sci. 2017, 20. [Google Scholar] [CrossRef]
- Vouillamoz, J.F.; Carlen, C.; Taglialatela-Scafati, O.; Pollastro, F.; Appendino, G. The génépi Artemisia species. Ethnopharmacology, cultivation, phytochemistry, and bioactivity. Fitoterapia 2015, 106, 231–241. [Google Scholar] [CrossRef]
- Rastogi, S.; Pandey, M.M.; Rawat, A.K.S. Medicinal plants of the genus Betula—Traditional uses and a phytochemical-pharmacological review. J. Ethnopharmacol. 2015, 159, 62–83. [Google Scholar] [CrossRef]
- WHO. WHO Monographs Selected Medicinal Plants; WHO Library Cataloguing in Publication Data: Geneva, Switzerland, 1999; Volume 1. [Google Scholar]
- Dinda, M.; Mazumdar, S.; Das, S.; Ganguly, D.; Dasgupta, U.B.; Dutta, A.; Jana, K.; Karmakar, P. The Water Fraction of Calendula officinalis Hydroethanol Extract Stimulates In Vitro and In Vivo Proliferation of Dermal Fibroblasts in Wound Healing. Phyther. Res. 2016, 30, 1696–1707. [Google Scholar] [CrossRef]
- Fonseca, Y.M.; Catini, C.D.; Vicentini, F.T.M.C.; Nomizo, A.; Gerlach, R.F.; Fonseca, M.J.V. Protective effect of Calendula officinalis extract against UVB-induced oxidative stress in skin: Evaluation of reduced glutathione levels and matrix metalloproteinase secretion. J. Ethnopharmacol. 2010, 127, 596–601. [Google Scholar] [CrossRef]
- Givol, O.; Kornhaber, R.; Visentin, D.; Cleary, M.; Haik, J.; Harats, M. A systematic review of Calendula officinalis extract for wound healing. Wound Repair Regen. 2019, 27, 548–561. [Google Scholar] [CrossRef]
- Nicolaus, C.; Junghanns, S.; Hartmann, A.; Murillo, R.; Ganzera, M.; Merfort, I. In vitro studies to evaluate the wound healing properties of Calendula officinalis extracts. J. Ethnopharmacol. 2017, 196, 94–103. [Google Scholar] [CrossRef]
- Buzzi, M.; De Freitas, F.; De Barros Winter, M. Therapeutic effectiveness of a Calendula officinalis extract in venous leg ulcer healing. J. Wound Care 2016, 25, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.; Deliorman-Orhan, D.; Özçelik, B. Antiviral activity and cytotoxicity of the lipophilic extracts of various edible plants and their fatty acids. Food Chem. 2009, 115, 701–705. [Google Scholar] [CrossRef]
- Kramer, P.; Wincierz, U.; Grubler, G.; Tschakert, J.; Voelter, W.; Mayer, H. Rational approach to fractionation, isolation, and characterization of polysaccharides from the lichen Cetraria islandica. Arzneim. Forsch. Drug Res. 1995, 45, 726–731. [Google Scholar]
- Freysdottir, J.; Omarsdottir, S.; Ingolfsdottir, K.; Vikingsson, A.; Olafsdottir, E.S. In vitro and in vivo immunomodulating effects of traditionally-prepared extract and purified compounds from Cetraria islandica. Proc. Nutr. Soc. 2008, 67, 423–430. [Google Scholar] [CrossRef] [Green Version]
- Olafsdottir, E.S.; Ingólfsdottir, K. Polysaccharides from lichens: Structural characteristics and biological activity. Planta Med. 2001, 67, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Kotan, E.; Alpsoy, L.; Anar, M.; Aslan, A.; Agar, G. Protective role of methanol extract of Cetraria islandica (L.) against oxidative stress and genotoxic effects of AFB(1) in human lymphocytes in vitro. Toxicol. Ind. Health 2011, 27, 599–605. [Google Scholar] [CrossRef]
- Nawrot, R. Defense-related Proteins from Chelidonium majus L. as Important Components of its Latex. Curr. Protein Pept. Sci. 2017, 18, 864–880. [Google Scholar] [CrossRef]
- Nawrot, J.; Wilk-jędrusik, M.; Nawrot, S.; Nawrot, K.; Wilk, B.; Dawid-Pać, R.; Urbańska, M.; Micek, I.; Nowak, G.; Gornowicz-porowska, J. Milky sap of greater celandine (Chelidonium majus L.) and anti-viral properties. Int. J. Environ. Res. Public Health 2020, 17, 1540. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.N.; Lin, Z.J.; Zhang, B.; Bai, Y.F. Effects of chicory on serum uric acid, renal function, and GLUT9 expression in hyperuricaemic rats with renal injury and in vitro verification with cells. Evid. Based Complement. Altern. Med. 2018, 2018, 1764212. [Google Scholar] [CrossRef] [Green Version]
- Emamiyan, M.Z.; Vaezi, G.; Tehranipour, M.; Shahrohkabadi, K.; Shiravi, A. Preventive effects of the aqueous extract of Cichorium intybus L. flower on ethylene glycol-induced renal calculi in rats. Avicenna J. Phytomed. 2018, 8, 170–178. [Google Scholar]
- Granica, S.; Piwowarski, J.P.; Czerwińska, M.E.; Kiss, A.K. Phytochemistry, pharmacology and traditional uses of different Epilobium species (Onagraceae): A review. J. Ethnopharmacol. 2014, 156, 316–346. [Google Scholar] [CrossRef] [PubMed]
- Vitalone, A.; Allkanjari, O. Epilobium spp: Pharmacology and Phytochemistry. Phyther. Res. 2018, 32, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Al-Snafi, A.E. The pharmacology of Equisetum arvense—A review. IOSR J. Pharm. 2017, 7, 31–42. [Google Scholar] [CrossRef]
- Asgarpanah, J.; Roohi, E. Phytochemistry and pharmacological properties of Equisetum arvense L. J. Med. Plants Res. 2012, 6. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, D.M.; Freire, R.C.; de Deus Honório, T.C.; Zoghaib, I.; Cardoso, F.F.; Tresvenzol, L.M.; de Paula, J.R.; Lima Sousa, A.L.; Brandão Veiga Jardimm, P.C.; da Cunha, L.C. Randomized, Double-Blind Clinical Trial to Assess the Acute Diuretic Effect of Equisetum arvense (Field Horsetail) in Healthy Volunteers. Evid. Based Complement. Altern. Med. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- do Monte, F.H.M.; dos Santos, J.G.; Russi, M.; Bispo Lanziotti, V.M.N.; Moreira Leal, L.K.A.; de Andrade Cunha, G.M. Antinociceptive and anti-inflammatory properties of the hydroalcoholic extract of stems from Equisetum arvense L. in mice. Pharmacol. Res. 2004, 49, 239–243. [Google Scholar] [CrossRef]
- Dragos, D.; Gilca, M.; Gaman, L.; Vlad, A.; Iosif, L.; Stoian, I.; Lupescu, O. Phytomedicine in Joint Disorders. Nutrients 2017, 9, 70. [Google Scholar] [CrossRef]
- Hedaya, R. Five herbs plus thiamine reduce pain and improve functional mobility in patients with pain: A pilot study. Altern. Ther. Health Med. 2017, 23, 14–19. [Google Scholar]
- Zgrajka, W.; Turska, M.; Rajtar, G.; Majdan, M.; Parada-Turska, J. Kynurenic acid content in anti-rheumatic herbs. Ann. Agric. Environ. Med. 2013, 20, 800–802. [Google Scholar]
- Bigagli, E.; Cinci, L.; D’Ambrosio, M.; Luceri, C. Pharmacological activities of an eye drop containing Matricaria chamomilla and Euphrasia officinalis extracts in UVB-induced oxidative stress and inflammation of human corneal cells. J. Photochem. Photobiol. B Biol. 2017, 173, 618–625. [Google Scholar] [CrossRef]
- Paduch, R.; Woźniak, A.; Niedziela, P.; Rejdak, R. Assessment of Eyebright (Euphrasia Officinalis L.) Extract Activity in Relation to Human Corneal Cells Using In Vitro Tests. Balkan Med. J. 2014, 33, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H. Foeniculum vulgare Mill: A Review of Its Botany, Phytochemistry, Pharmacology, Contemporary Application, and Toxicology. Biomed. Res. Int. 2014, 2014, 1–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portincasa, P.; Bonfrate, L.; Scribano, M.L.; Kohn, A.; Caporaso, N.; Festi, D.; Campanale, M.C.; Di Rienzo, T.; Guarino, M.; Taddia, M.; et al. Curcumin and fennel essential oil improve symptoms and quality of life in patients with irritable bowel syndrome. J. Gastrointest. Liver Dis. 2016, 25, 151–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niiho, Y.; Yamazaki, T.; Nakajima, Y.; Yamamoto, T.; Ando, H.; Hirai, Y.; Toriizuka, K.; Ida, Y. Gastroprotective effects of bitter principles isolated from Gentian root and Swertia herb on experimentally-induced gastric lesions in rats. J. Nat. Med. 2006, 60, 82–88. [Google Scholar] [CrossRef]
- Wölfle, U.; Seelinger, G.; Schempp, C.M. Topical application of St John’s wort (Hypericum perforatum). Planta Med. 2014, 80, 109–120. [Google Scholar]
- Uslusoy, F.; Nazıroğlu, M.; Övey, İ.S.; Sönmez, T.T. Hypericum perforatum L. supplementation protects sciatic nerve injury-induced apoptotic, inflammatory and oxidative damage to muscle, blood and brain in rats. J. Pharm. Pharmacol. 2019, 71, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Di Pierro, F.; Risso, P.; Settembre, R. Role in depression of a multi-fractionated versus a conventional Hypericum perforatum extract. Panminerva Med. 2018, 60. [Google Scholar] [CrossRef]
- Eatemadnia, A.; Ansari, S.; Abedi, P.; Najar, S. The effect of Hypericum perforatum on postmenopausal symptoms and depression: A randomized controlled trial. Complement. Ther. Med. 2019, 45, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Venkatanarayanan, N.; Ho, C.Y.X. Clinical use of Hypericum perforatum (St John’s wort) in depression: A meta-analysis. J. Affect. Disord. 2017, 210, 211–221. [Google Scholar] [CrossRef]
- Majewska, E.; Kozłowska, M.; Kowalska, D.; Gruczyńska, E. Characterization of the essential oil from cone-berries of Juniperus communis L. (Cupressaceae). Herba Pol. 2017, 63, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Raina, R.; Verma, P.K.; Peshin, R.; Kour, H. Potential of Juniperus communis L as a nutraceutical in human and veterinary medicine. Heliyon 2019, 5, e02376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kılıç, Ö.; Kocak, A. Volatile Constituents of Juniperus communis L., Taxus canadensis Marshall. and Tsuga canadensis (L.) Carr. from Canada. J. Agric. Sci. Technol. B 2014, 4, 135–140. [Google Scholar]
- Bahmani, M.; Zargaran, A. Ethno-botanical medicines used for urinary stones in the Urmia, Northwest Iran. Eur. J. Integr. Med. 2015, 7, 657–662. [Google Scholar] [CrossRef]
- Matkowski, A.; Piotrowska, M. Antioxidant and free radical scavenging activities of some medicinal plants from the Lamiaceae. Fitoterapia 2006, 77, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Cardia, G.F.E.; Silva-Filho, S.E.; Silva, E.L.; Uchida, N.S.; Cavalcante, H.A.O.; Cassarotti, L.L.; Salvadego, V.E.C.; Spironello, R.A.; Bersani-Amado, C.A.; Cuman, R.K.N. Effect of Lavender (Lavandula angustifolia) Essential Oil on Acute Inflammatory Response. Evid. Based Complement. Altern. Med. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Lavandula angustifolia and Lavandula latifolia Essential Oils from Spain: Aromatic Profile and Bioactivities. Planta Med. 2015, 82, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Giovannini, D.; Gismondi, A.; Basso, A.; Canuti, L.; Braglia, R.; Canini, A.; Mariani, F.; Cappelli, G. Lavandula angustifolia Mill. Essential oil exerts antibacterial and anti-inflammatory effect in macrophage mediated immune response to Staphylococcus aureus. Immunol. Investig. 2016, 45, 11–28. [Google Scholar] [CrossRef]
- Tagreed Altaei, D. Topical lavender oil for the treatment of recurrent aphthous ulceration. Am. J. Dent. 2012, 25, 39–43. [Google Scholar]
- Nasiri Lari, Z.; Hajimonfarednejad, M.; Riasatian, M.; Abolhassanzadeh, Z.; Iraji, A.; Vojoud, M.; Heydari, M.; Shams, M. Efficacy of inhaled Lavandula angustifolia Mill. Essential oil on sleep quality, quality of life and metabolic control in patients with diabetes mellitus type II and insomnia. J. Ethnopharmacol. 2020, 251, 112560. [Google Scholar] [CrossRef]
- Pferschy-Wenzig, E.M.; Kunert, O.; Presser, A.; Bauer, R. In Vitro Anti-inflammatory Activity of Larch (Larix decidua L.) Sawdust. J. Agric. Food Chem. 2008, 56, 11688–11693. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Elansary, H.O.; Elkelish, A.A.; Zeidler, A.; Ali, H.M.; Hefny, M.E.L.; Yessoufou, K. In vitro bioactivity and antimicrobial activity of Picea abies and Larix decidua wood and bark extracts. BioResources 2016, 11, 9421–9437. [Google Scholar] [CrossRef] [Green Version]
- Afifi, F.U.; Khalil, E.; Tamimi, S.O.; Disi, A. Evaluation of the gastroprotective effect of Laurus nobilis seeds on ethanol induced gastric ulcer in rats. J. Ethnopharmacol. 1997, 58, 9–14. [Google Scholar] [CrossRef]
- Gürbüz, İ.; Üstün, O.; Yeşilada, E.; Sezik, E.; Akyürek, N. In vivo gastroprotective effects of five Turkish folk remedies against ethanol-induced lesions. J. Ethnopharmacol. 2002, 83, 241–244. [Google Scholar] [CrossRef]
- Speroni, E.; Cervellati, R.; Dall’Acqua, S.; Guerra, M.C.; Greco, E.; Govoni, P.; Innocenti, G. Gastroprotective effect and antioxidant properties of different Laurus nobilis L. leaf extracts. J. Med. Food 2011, 14, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Motti, R.; Bonanomi, G.; Emrick, S.; Lanzotti, V. Traditional Herbal Remedies Used in women’s Health Care in Italy: A Review. Hum. Ecol. 2019, 47, 941–972. [Google Scholar] [CrossRef]
- Patrakar, R.; Mansuriya, M.; Patil, P. Phytochemical and Pharmacological Review on Laurus nobilis. Int. J. Pharm. Chem. Sci. 2012, 1, 595–602. [Google Scholar]
- Xu, J.; Zhou, X.; Chen, C.; Deng, Q.; Huang, Q.; Yang, J.; Yang, N.; Huang, F. Laxative effects of partially defatted flaxseed meal on normal and experimental constipated mice. BMC Complement. Altern. Med. 2012, 12. [Google Scholar] [CrossRef] [Green Version]
- Hanif Palla, A.; Gilani, A.-H. Dual effectiveness of Flaxseed in constipation and diarrhea: Possible mechanism. J. Ethnopharmacol. 2015, 169, 60–68. [Google Scholar] [CrossRef]
- European Medicine Agency. Available online: https://www.ema.europa.eu/en/documents/herbal-summary/linseed-summary-public_en.pdf (accessed on 5 February 2020).
- Saadat, S.; Shakeri, F.; Boskabady, M.H. Comparative Antitussive Effects of Medicinal Plants and Their Constituents. Altern. Ther. 2018, 24, 36–49. [Google Scholar]
- Benso, B.; Rosalen, P.L.; Alencar, S.M.; Murata, R.M. Malva sylvestris Inhibits Inflammatory Response in Oral Human Cells. An In Vitro Infection Model. PLoS ONE 2015, 10, e0140331. [Google Scholar] [CrossRef] [Green Version]
- Braga, A.S.; Pires, J.G.; Magalhães, A.C. Effect of a mouthrinse containing Malva sylvestris on the viability and activity of microcosm biofilm and on enamel demineralization compared to known antimicrobials mouthrinses. Biofouling 2018, 34, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Gasparetto, J.C.; Martins, C.A.F.; Hayashi, S.S.; Otuky, M.F.; Pontarolo, R. Ethnobotanical and scientific aspects of Malva sylvestris L.: A millennial herbal medicine. J. Pharm. Pharmacol. 2012, 64, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Campos, M.; Irioda, A.; Stremel, D.; Trindade, A.; Pontarolo, R. Anti-Inflammatory Effect of Malva sylvestris, Sida cordifolia, and Pelargonium graveolens Is Related to Inhibition of Prostanoid Production. Molecules 2017, 22, 1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahimi, S.; Abdollahi, M.; Mortazavi, S.A.; Hajimehdipoor, H.; Abdolghaffari, A.H.; Rezvanfar, M.A. Wound Healing Activity of a Traditionally Used Poly Herbal Product in a Burn Wound Model in Rats. Iran. Red Crescent Med. J. 2015, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirbalouti, A.G.; Shahrzad, A.; Abed, K.; Hamedi, B. Wound healing activity of Malva sylvestris and Punica granatum in alloxan-induced diabetic rats. Acta Pol. Pharm. Drug Res. 2010, 67, 511–516. [Google Scholar]
- Prudente, A.S.; Sponchiado, G.; Mendes, D.A.G.B.; Soley, B.S.; Cabrini, D.A.; Otuki, M.F. Pre-clinical efficacy assessment of Malva sylvestris on chronic skin inflammation. Biomed. Pharmacother. 2017, 93, 852–860. [Google Scholar] [CrossRef]
- Prudente, A.S.; Loddi, A.M.V.; Duarte, M.R.; Santos, A.R.S.; Pochapski, M.T.; Pizzolatti, M.G.; Hayashi, S.S.; Campos, F.R.; Pontarolo, R.; Santos, F.A.; et al. Pre-clinical Anti-Inflammatory Aspects of a Cuisine and Medicinal Millennial Herb: Malva sylvestris L. Food Chem. Toxicol. 2013, 58, 324–331. [Google Scholar] [CrossRef]
- Marouane, W.; Soussi, A.; Murat, J.-C.; Bezzine, S.; El Feki, A. The protective effect of Malva sylvestris on rat kidney damaged by vanadium. Lipids Health Dis. 2011, 10, 65. [Google Scholar] [CrossRef] [Green Version]
- Mohamadi Yarijani, Z.; Najafi, H.; Shackebaei, D.; Madani, S.H.; Modarresi, M.; Jassemi, S.V. Amelioration of renal and hepatic function, oxidative stress, inflammation and histopathologic damages by Malva sylvestris extract in gentamicin induced renal toxicity. Biomed. Pharmacother. 2019, 112, 108635. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.B. A Review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phyther. Res. 2006, 20, 519–530. [Google Scholar] [CrossRef]
- Miraj, S.; Alesaeidi, S. A systematic review study of therapeutic effects of Matricaria recuitta chamomile (chamomile). Electron. Physician 2016, 8, 3024–3031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehmood, M.H.; Munir, S.; Khalid, U.A.; Asrar, M.; Gilani, A.H. Antidiarrhoeal, antisecretory and antispasmodic activities of Matricaria chamomilla are mediated predominantly through K+-channels activation. BMC Complement. Altern. Med. 2015, 15, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keefe, J.R.; Mao, J.J.; Soeller, I.; Li, Q.S.; Amsterdam, J.D. Short-term open-label chamomile (Matricaria chamomilla L.) therapy of moderate to severe generalized anxiety disorder. Phytomedicine 2016, 23, 1699–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, J.J.; Xie, S.X.; Keefe, J.R.; Soeller, I.; Li, Q.S.; Amsterdam, J.D. Long-term chamomile (Matricaria chamomilla L.) treatment for generalized anxiety disorder: A randomized clinical trial. Phytomedicine 2016, 23, 1735–1742. [Google Scholar] [CrossRef] [Green Version]
- Akbarzadeh, M.; Dehghani, M.; Moshfeghy, Z.; Emamghoreishi, M.; Tavakoli, P.; Zare, N. Effect of Melissa officinalis Capsule on the Intensity of Premenstrual Syndrome Symptoms in High School Girl Students. Nurs. Midwifery Stud. 2015, 4, 24–28. [Google Scholar] [CrossRef]
- Mirabi, P.; Namdari, M.; Alamolhoda, S.H.; Mojab, F. The effect of Melissa officinalis extract on the severity of primary dysmenorrhea. Iran. J. Pharm. Res. 2017, 16, 171–177. [Google Scholar] [CrossRef]
- Atta, A.H.; Alkofahi, A. Anti-nociceptive and anti-inflammatory effects of some Jordanian medicinal plant extracts. J. Ethnopharmacol. 1998, 60, 117–124. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, H.; Wang, J.; Zhou, L.; Yang, P. Chemical Composition and Anti-Inflammatory, Cytotoxic and Antioxidant Activities of Essential Oil from Leaves of Mentha piperita Grown in China. PLoS ONE 2014, 9, e114767. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.X.; Liu, Y.B.; Ma, A.Q.; Bao, Y.; Wang, M.; Sun, Z.L. In vitro antiviral, anti-inflammatory, and antioxidant activities of the ethanol extract of Mentha piperita L. Food Sci. Biotechnol. 2017, 26, 1675–1683. [Google Scholar] [CrossRef]
- Silva, F.V.; Guimarães, A.G.; Silva, E.R.S.; Sousa-Neto, B.P.; MacHado, F.D.F.; Quintans-Júnior, L.J.; Arcanjo, D.D.R.; Oliveira, F.A.; Oliveira, R.C.M. Anti-Inflammatory and Anti-Ulcer Activities of Carvacrol, a Monoterpene Present in the Essential Oil of Oregano. J. Med. Food 2012, 15, 984–991. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, B.; Liu, J.; Ma, X. Carvacrol ameliorates inflammatory response in interleukin 1β-stimulated human chondrocytes. Mol. Med. Rep. 2017, 17, 3987–3992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naquvi, K.J.; Ahamad, J.; Salma, A.; Ansari, S.H.; Najmi, A.K. A critical review on traditional uses, phytochemistry, and pharmacological uses of Origanum vulgare Linn. Int. Res. J. Pharm. 2019, 10, 7–11. [Google Scholar] [CrossRef]
- Singh, P.; Kothiyal, P.; Ratan, P. Pharmacological and Phytochemical Studies of Origanum vulgare: A Review. Int. Res. J. Pharm. 2018, 9, 30–34. [Google Scholar] [CrossRef]
- Guerrero, F.A.; Medina, G.M. Effect of a medicinal plant (Passiflora incarnata L) on sleep. Sleep Sci. 2017, 10, 96–100. [Google Scholar] [CrossRef]
- Kim, G.; Kim, Y.; Yoon, S.; Kim, S.; Yi, S.S. Sleep-inducing effect of Passiflora incarnata L. extract by single and repeated oral administration in rodent animals. Food Sci. Nutr. 2020, 8, 557–566. [Google Scholar] [CrossRef]
- Kim, M.; Lim, H.-S.; Lee, H.-H.; Kim, T.-H. Role Identification of Passiflora incarnata Linnaeus: A Mini Review. J. Menopausal Med. 2017, 23, 156. [Google Scholar] [CrossRef] [Green Version]
- Miroddi, M.; Calapai, G.; Navarra, M.; Minciullo, P.L.; Gangemi, S. Passiflora incarnata L.: Ethnopharmacology, clinical application, safety and evaluation of clinical trials. J. Ethnopharmacol. 2013, 150, 791–804. [Google Scholar] [CrossRef]
- Ngan, A.; Conduit, R. A double-blind, placebo-controlled investigation of the effects of Passiflora incarnata (passionflower) herbal tea on subjective sleep quality. Phyther. Res. 2011, 25, 1153–1159. [Google Scholar] [CrossRef]
- Haddadian, K.K.; Haddadian, K.K.; Zahmatkash, M. A review of Plantago plant. Indian J. Tradit Know 2014, 13, 5. [Google Scholar]
- Miraj, S. A review study of pharmacological properties of Plantago major l. Der Pharma Chem. 2016, 8, 21–25. [Google Scholar]
- Adom, M.B.; Taher, M.; Mutalabisin, M.F.; Amri, M.S.; Abdul Kudos, M.B.; Wan Sulaiman, M.W.A.; Sengupta, P.; Susanti, D. Chemical constituents and medical benefits of Plantago major. Biomed. Pharmacother. 2017, 96, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Najafian, Y.; Hamedi, S.S.; Kaboli Farshchi, M.; Feyzabadi, Z. Plantago major in Traditional Persian Medicine and modern phytotherapy: A narrative review. Electron. Physician 2018, 10, 6390–6399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fyhrquist, P.; Virjamo, V.; Hiltunen, E.; Julkunen-Tiitto, R. Epidihydropinidine, the main piperidine alkaloid compound of Norway spruce (Picea abies) shows promising antibacterial and anti-Candida activity. Fitoterapia 2017, 117, 138–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tunón, H.; Olavsdotter, C.; Bohlin, L. Evaluation of anti-inflammatory activity of some Swedish medicinal plants. Inhibition of prostaglandin biosynthesis and PAF-induced exocytosis. J. Ethnopharmacol. 1995, 48, 61–76. [Google Scholar] [CrossRef]
- Apetrei, C.L.; Tuchilus, C.; Aprotosoaie, A.C.; Oprea, A.; Malterud, K.E.; Miron, A.; Aprotosaie, A.C.; Oprea, A.; Malterud, K.E.; Miron, A. Chemical, Antioxidant and Antimicrobial Investigations of Pinus cembra L. Bark and Needles. Molecules 2011, 16, 7773–7788. [Google Scholar] [CrossRef] [Green Version]
- Basholli-Salihu, M.; Schuster, R.; Hajdari, A.; Mulla, D.; Viernstein, H.; Mustafa, B.; Mueller, M. Phytochemical composition, anti-inflammatory activity and cytotoxic effects of essential oils from three Pinus spp. Pharm. Biol. 2017, 55, 1553–1560. [Google Scholar] [CrossRef] [Green Version]
- Mitić, Z.S.; Jovanović, B.; Jovanović, S.Č.; Mihajilov-Krstev, T.; Stojanović-Radić, Z.Z.; Cvetković, V.J.; Mitrović, T.L.; Marin, P.D.; Zlatković, B.K.; Stojanović, G.S. Comparative study of the essential oils of four Pinus species: Chemical composition, antimicrobial and insect larvicidal activity. Ind. Crops Prod. 2018, 111, 55–62. [Google Scholar] [CrossRef]
- Ciuman, R.R. Phytotherapeutic and naturopathic adjuvant therapies in otorhinolaryngology. Eur. Arch. Oto Rhino Laryngology 2012, 269, 389–397. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency Evaluation of Medicines for Human Use. EMEA Assessment Report on Polypodium vulgare L., RHIZOMA; European Medicines Agency: London, UK, 2008. [Google Scholar]
- Hooman, N.; Mojab, F.; Nickavar, B.; Pouryousefi-Kermani, P. Diuretic effect of powdered Cerasus avium (cherry) tails on healthy volunteers. Pak. J. Pharm. Sci. 2009, 22, 381–383. [Google Scholar]
- Selahvarzian, A.; Alizadeh, A.; Baharvand, P.A.; Eldahshan, O.A.; Rasoulian, B. Medicinal Properties of Rosa canina L. Herb. Med. J. 2018, 3, 77–84. [Google Scholar] [CrossRef]
- Winther, K.; Campbell-Tofte, J.; Vinther Hansen, A.S. Bioactive ingredients of rose hips (Rosa canina L.) with special reference to antioxidative and anti-inflammatory properties: In vitro studies. Bot. Targets Ther. 2016, 6, 11–23. [Google Scholar] [CrossRef] [Green Version]
- Al-Sereiti, M.R.; Abu-Amer, K.M.; Sen, P. Pharmacology of rosemary (Rosmarinus officinalis Linn.) and its therapeutic potentials. Indian J. Exp. Biol. 1999, 37, 124–130. [Google Scholar] [PubMed]
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement. Altern. Med. 2014, 14. [Google Scholar] [CrossRef] [Green Version]
- Hassani, F.V.; Shirani, K.; Hosseinzadeh, H. Rosemary (Rosmarinus officinalis) as a potential therapeutic plant in metabolic syndrome: A review. Naunyn. Schmiedebergs. Arch. Pharmacol. 2016, 389, 931–949. [Google Scholar] [CrossRef]
- Hegazy, A.M.; Abdel-Azeem, A.S.; Zeidan, H.M.; Ibrahim, K.S.; El Sayed, E.M. Hypolipidemic and hepatoprotective activities of rosemary and thyme in gentamicin-treated rats. Hum. Exp. Toxicol. 2018, 37, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rodríguez, J.L.; Gutiérrez-Hernández, R.; Reyes-Estrada, C.A.; Granados-López, A.J.; Pérez-Veyna, O.; Arcos-Ortega, T.; López, J.A. Hepatoprotective, antihyperlipidemic and radical scavenging activity of hawthorn (Crataegus oxyacantha) and rosemary (Rosmarinus officinalis) on alcoholic liver disease. Altern. Ther. Health Med. 2019, 25, 54–63. [Google Scholar]
- WHO. WHO Monographs on Selected Medicinal Plants; WHO Library Cataloguing in Publication Data: Geneva, Switzerland, 2009; Volume 4. [Google Scholar]
- Ozturk, S.; Ozturk, A. Antibacterial Activity of Aqueous and Methanol Extracts of Rumex alpinus and Rumex caucasicus. Pharm. Biol. 2007, 45, 83–87. [Google Scholar] [CrossRef]
- Mishra, A.P.; Sharifi-Rad, M.; Shariati, M.A.; Mabkhot, Y.N.; Al-Showiman, S.S.; Rauf, A.; Salehi, B.; Župunski, M.; Sharifi-Rad, M.; Gusain, P.; et al. Bioactive compounds and health benefits of edible Rumex species-A review. Cell. Mol. Biol. 2018, 64, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Ehrnhöfer-Ressler, M.M.; Fricke, K.; Pignitter, M.; Walker, J.M.; Walker, J.; Rychlik, M.; Somoza, V. Identification of 1,8-Cineole, Borneol, Camphor, and Thujone as Anti-inflammatory Compounds in a Salvia officinalis L. Infusion Using Human Gingival Fibroblasts. J. Agric. Food Chem. 2013, 61, 3451–3459. [Google Scholar] [CrossRef]
- Lemle, K.L. Salvia officinalis used in pharmaceutics. Proc. IOP Conf. Ser. Mater. Sci. Eng. 2018, 294, 012037. [Google Scholar] [CrossRef]
- Jakovljević, M.; Jokić, S.; Molnar, M.; Jašić, M.; Babić, J.; Jukić, H.; Banjari, I. Bioactive Profile of Various Salvia officinalis L. Preparations. Plants 2019, 8, 55. [Google Scholar] [CrossRef] [Green Version]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Picon, P.D.; Picon, R.V.; Costa, A.F.; Sander, G.B.; Amaral, K.M.; Aboy, A.L.; Henriques, A.T. Randomized clinical trial of a phytotherapic compound containing Pimpinella anisum, Foeniculum vulgare, Sambucus nigra, and Cassia augustifolia for chronic constipation. BMC Complement. Altern. Med. 2010, 10, 17. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Zuckerman, D.M.; Brantley, S.; Sharpe, M.; Childress, K.; Hoiczyk, E.; Pendleton, A.R. Sambucus nigra extracts inhibit infectious bronchitis virus at an early point during replication. BMC Vet. Res. 2014, 10, 12. [Google Scholar] [CrossRef] [Green Version]
- Porter, R.S.; Bode, R.F. A Review of the Antiviral Properties of Black Elder (Sambucus nigra L.) Products: Antiviral Properties of Black Elder (Sambucus nigra L.). Phyther. Res. 2017, 31, 533–554. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, J.; Baker, C.; Cherry, L.; Dunne, E. Black elderberry (Sambucus nigra) supplementation effectively treats upper respiratory sympoms: A meta-analysis of randomized, controlled clinical trials. Complement. Ther. Med. 2019, 42, 361–365. [Google Scholar] [CrossRef]
- Bahmani, M.; Shirzad, H.; Rafieian, S.; Rafieian-Kopaei, M. Silybum marianum: Beyond Hepatoprotection. J. Evid. Based Complement. Altern. Med. 2015, 20, 292–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazazis, C.; Geladari, E.; Trigkidis, K.; Vallianou, N.G. Milk Thistle: New Perspectives for an Old Remedy. Curr. Top. Nutraceut. Res. 2016, 14, 103–107. [Google Scholar]
- Kenny, O.M.; McCarthy, C.M.; Brunton, N.P.; Hossain, M.B.; Rai, D.K.; Collins, S.G.; Jones, P.W.; Maguire, A.R.; O’Brien, N.M. Anti-inflammatory properties of potato glycoalkaloids in stimulated Jurkat and Raw 264.7 mouse macrophages. Life Sci. 2013, 92, 775–782. [Google Scholar] [CrossRef]
- Visvanathan, R.; Jayathilake, C.; Chaminda Jayawardana, B.; Liyanage, R. Health-beneficial properties of potato and compounds of interest: Health-beneficial properties of potato. J. Sci. Food Agric. 2016, 96, 4850–4860. [Google Scholar] [CrossRef]
- Basilicata, M.G.; Pepe, G.; Rapa, S.F.; Merciai, F.; Ostacolo, C.; Manfra, M.; Di Sarno, V.; Autore, G.; De Vita, D.; Marzocco, S.; et al. Anti-Inflammatory and Antioxidant Properties of Dehydrated Potato-Derived Bioactive Compounds in Intestinal Cells. Int. J. Mol. Sci. 2019, 20, 6087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popovych, V.; Koshel, I.; Malofiichuk, A.; Pyletska, L.; Semeniuk, A.; Filippova, O.; Orlovska, R. A randomized, open-label, multicenter, comparative study of therapeutic efficacy, safety and tolerability of BNO 1030 extract, containing marshmallow root, chamomile flowers, horsetail herb, walnut leaves, yarrow herb, oak bark, dandelion herb in the trea. Am. J. Otolaryngol 2019, 40, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Fatima, T.; Bashir, O.; Naseer, B.; Hussain, S.Z. Dandelion: Phytochemistry and clinical potential. J. Med. Plants Stud. 2018, 6, 198–202. [Google Scholar]
- Jin, Y.R.; Jin, J.; Piao, X.X.; Jin, N.G. The effect of Taraxacum officinale on gastric emptying and smooth muscle motility in Rodents. Neurogastroenterol. Motil. 2011, 23. [Google Scholar] [CrossRef]
- Martinez, M.; Poirrier, P.; Chamy, R.; Prüfer, D.; Schulze-Gronover, C.; Jorquera, L.; Ruiz, G. Taraxacum officinale and related species—An ethnopharmacological review and its potential as a commercial medicinal plant. J. Ethnopharmacol. 2015, 169, 244–262. [Google Scholar] [CrossRef]
- González-Castejón, M.; Visioli, F.; Rodriguez-Casado, A. Diverse biological activities of dandelion. Nutr. Rev. 2012, 70, 534–547. [Google Scholar] [CrossRef]
- Schütz, K.; Carle, R.; Schieber, A. Taraxacum—A review on its phytochemical and pharmacological profile. J. Ethnopharmacol. 2006, 107, 313–323. [Google Scholar] [CrossRef]
- Lans, C.; Turner, N.; Khan, T.; Brauer, G.; Boepple, W. Ethnoveterinary medicines used for ruminants in British Columbia, Canada. J. Ethnobiol. Ethnomed. 2007, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Ayrle, H.; Mevissen, M.; Kaske, M.; Nathues, H.; Gruetzner, N.; Melzig, M.; Walkenhorst, M. Medicinal plants—Prophylactic and therapeutic options for gastrointestinal and respiratory diseases in calves and piglets? A systematic review. BMC Vet. Res. 2016, 12, 89. [Google Scholar] [CrossRef] [Green Version]
- Jarić, S.; Mitrović, M.; Pavlović, P. Review of Ethnobotanical, Phytochemical, and Pharmacological Study of Thymus serpyllum L. Evid. Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Prakash Mishra, A.; Shukla, I.; Sharifi-Rad, M.; Del Mar Contreras, M.; Segura-Carretero, A.; Fathi, H.; Nasri Nasrabasi, N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, Thyme, and Other Plant Sources: Health and Potential Uses. Phyther. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, F.; Ghorani, V.; Saadat, S.; Gholamnezhad, Z.; Boskabady, M.H. The stimulatory effects of medicinal plants on β2-adrenoceptors of tracheal smooth muscle. Iran. J. Allergy, Asthma Immunol. 2019, 18, 12–26. [Google Scholar] [CrossRef] [Green Version]
- Asgarpanah, J.; Mohajerani, R. Phytochemistry and pharmacologic properties of Urtica dioica L. J. Med. Plants Res. 2012, 6, 5714–5719. [Google Scholar] [CrossRef]
- Joshi, B.C.; Mukhija, M.; Kalia, A.N. Pharmacognostical review of Urtica dioica L. Int. J. Green Pharm. 2014, 8, 201–209. [Google Scholar] [CrossRef]
- Ahmed KK, M.; Parsuraman, S. Urtica dioica L., (Urticaceae): A stinging nettle. Syst. Rev. Pharm. 2014, 5, 6–8. [Google Scholar] [CrossRef]
- Kregiel, D.; Pawlikowska, E.; Antolak, H. Urtica spp.: Ordinary Plants with Extraordinary Properties. Molecules 2018, 23, 1664. [Google Scholar] [CrossRef] [Green Version]
- Deng, H.W.; Tian, Y.; Zhou, X.J.; Zhang, X.M.; Meng, J. Effect of Bilberry Extract on Development of Form-Deprivation Myopia in the Guinea Pig. J. Ocul. Pharmacol. Ther. 2016, 32, 196–202. [Google Scholar] [CrossRef]
- Gizzi, C.; Belcaro, G.; Gizzi, G.; Feragalli, B.; Dugall, M.; Luzzi, R.; Cornelli, U. Bilberry extracts are not created equal: The role of non anthocyanin fraction. Discovering the “dark side of the force” in a preliminary study. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2418–2424. [Google Scholar]
- Matsunaga, N.; Imai, S.; Inokuchi, Y.; Shimazawa, M.; Yokota, S.; Araki, Y.; Hara, H. Bilberry and its main constituents have neuroprotective effects against retinal neuronal damage in vitro and in vivo. Mol. Nutr. Food Res. 2009, 53, 869–877. [Google Scholar] [CrossRef]
- Ogawa, K.; Tsuruma, K.; Tanaka, J.; Kakino, M.; Kobayashi, S.; Shimazawa, M.; Hara, H. The Protective Effects of Bilberry and Lingonberry Extracts against UV Light-Induced Retinal Photoreceptor Cell Damage in Vitro. J. Agric. Food Chem. 2013, 61, 10345–10353. [Google Scholar] [CrossRef]
- Ogawa, K.; Kuse, Y.; Tsuruma, K.; Kobayashi, S.; Shimazawa, M.; Hara, H. Protective effects of bilberry and lingonberry extracts against blue light-emitting diode light-induced retinal photoreceptor cell damage in vitro. BMC Complement. Altern. Med. 2014, 14, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozawa, Y.; Kawashima, M.; Inoue, S.; Inagaki, E.; Suzuki, A.; Ooe, E.; Kobayashi, S.; Tsubota, K. Bilberry extract supplementation for preventing eye fatigue in video display terminal workers. J. Nutr. Health Aging 2015, 19, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Szajdek, A.; Borowska, E.J. Bioactive Compounds and Health-Promoting Properties of Berry Fruits: A Review. Plant. Foods Hum. Nutr. 2008, 63, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, L.; Lu, F.; Yang, X.; Deng, Q.; Ji, B.; Huang, F.; Kitts, D.D. Retinoprotective effects of bilberry anthocyanins via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms in a visible light-induced retinal degeneration model in pigmented rabbits. Molecules 2015, 20, 22395–22410. [Google Scholar] [CrossRef] [Green Version]
- Canter, P.H.; Ernst, E. Anthocyanosides of Vaccinium myrtillus (bilberry) for night vision—A systematic review of placebo-controlled trials. Surv. Ophthalmol. 2004, 49, 38–50. [Google Scholar] [CrossRef]
- Persson, I.A.-L.; Persson, K.; Andersson, R.G.G. Effect of Vaccinium myrtillus and Its Polyphenols on Angiotensin-Converting Enzyme Activity in Human Endothelial Cells. J. Agric. Food Chem. 2009, 2009, 4626–4629. [Google Scholar] [CrossRef]
- Ştefănescu, B.E.; Szabo, K.; Mocan, A.; Crişan, G. Phenolic Compounds from Five Ericaceae Species Leaves and Their Related Bioavailability and Health Benefits. Molecules 2019, 24, 2046. [Google Scholar] [CrossRef] [Green Version]
- Davidson, E.; Zimmermann, B.F.; Jungfer, E.; Chrubasik-Hausmann, S. Prevention of Urinary Tract Infections with Vaccinium Products. Phyther. Res. 2014, 28, 465–470. [Google Scholar] [CrossRef]
- Abdellah, S.A.; Berlin, A.; Blondeau, C.; Guinobert, I.; Guilbot, A.; Beck, M.; Duforez, F. A combination of Eschscholtzia californica Cham. and Valeriana officinalis L. extracts for adjustment insomnia: A prospective observational study. J. Tradit. Complement. Med. 2020, 10, 116–123. [Google Scholar] [CrossRef]
- Aliakbari, F.; Rafieian, M. The effectiveness of Valeriana officinalis on sleep disturbance in patients with chronic heart failure. Int. J. Pharm. Investig. 2018, 8, 145–150. [Google Scholar] [CrossRef]
- Bent, S.; Padula, A.; Moore, D.; Patterson, M.; Mehling, W. Valerian for Sleep: A Systematic Review and Meta-Analysis. Am. J. Med. 2006, 119, 1005–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mineo, L.; Concerto, C.; Patel, D.; Mayorga, T.; Paula, M.; Chusid, E.; Aguglia, E.; Battaglia, F. Valeriana officinalis Root Extract Modulates Cortical Excitatory Circuits in Humans. Neuropsychobiology 2017, 75, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Kubin, Z.J.; Shepherd, J.N.; Ettinger, R.H. Valeriana officinalis root extractshavepotentanxiolyticeffectsin laboratoryrats. Phytomedicine 2010, 17, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Murti, K.; Kaushik, M.; Sangwan, Y.; Kaushik, A. Pharmacological Properties of Valeriana officinalis—A Review. Pharmacologyonline 2011, 3, 641–646. [Google Scholar]
- Nandhini, S.; Narayanan, K.B.; Ilango, K. Valeriana officinalis: A Review of its Traditional Uses, Phytochemistry, and Pharmacology. Asian J. Pharm. Clin. Res. 2018, 11, 36. [Google Scholar] [CrossRef] [Green Version]
- Leach, M.J.; Page, A.T. Herbal medicine for insomnia: A systematic review and meta-analysis. Sleep Med. Rev. 2015, 24, 1–12. [Google Scholar] [CrossRef]
- Kurti, F.; Giorgi, A.; Beretta, G.; Mustafa, B.; Gelmini, F.; Testa, C.; Angioletti, S.; Giupponi, L.; Zilio, E.; Pentimalli, D.; et al. Chemical composition, antioxidant and antimicrobial activities of essential oils of different Pinus species from Kosovo. J. Essent. Oil Res. 2019, 31, 263–275. [Google Scholar] [CrossRef]
- Venditti, A.; Serrilli, A.M.; Vittori, S.; Papa, F.; Maggi, F.; Di Cecco, M.; Ciaschetti, G.; Bruno, M.; Rosselli, S.; Bianco, A. Secondary metabolites from Pinus mugo Turra subsp. mugo growing in the Majella National Park (Central Apennines, Italy). Chem. Biodivers. 2013, 10, 2091–2100. [Google Scholar] [CrossRef]
- Aziz, M.A.; Adnan, M.; Khan, A.H.; Shahat, A.A.; Al-Said, M.S.; Ullah, R. Traditional uses of medicinal plants practiced by the indigenous communities at Mohmand Agency, FATA, Pakistan. J. Ethnobiol. Ethnomed. 2018, 14, 2. [Google Scholar] [CrossRef] [Green Version]
- Pignatti, S.; Guorino, R.; La Rosa, M. Flora d’Italia, 2nd ed.; Edagricole, Ed.; New Business Media: Bologna, Italy, 2017. [Google Scholar]
- Signorini, M.A.; Lombardini, C.; Bruschi, P.; Vivona, L. Conoscenze etnobotaniche e saperi tradizionali nel territorio di San Miniato (Pisa). Atti. Soc. Tosc. Sci. Nat. Mem. Series B 2008, 1, 65–83. [Google Scholar]
- Trotter, R.T.; Logan, M.H. Informant Consensus: A New Approach for Identifying Potentially Effective Medicinalplants. In Plants in Indigenous Medicine & Diet: Biobehavioral Approaches; Etkin, N.L., Ed.; Redgrave Publishing Company: Bedford Hills/New York, NY, USA, 1986; pp. 91–112. [Google Scholar]
- Friedman, J.; Zohara, Y.; Amotz, D.; Palewitch, D. A preliminary classification of the healing potential of medicinal plants, based on a rational analysis of an ethnopharmacological field survey among Bedouins in the Negev Desert, Israel. J. Ethnopharmacol. 1986, 16, 275–278. [Google Scholar] [CrossRef]
- Mustafa, B.; Hajdari, A.; Pieroni, A.; Pulaj, B.; Koro, X.; Quave, C.L. A cross-cultural comparison of folk plant uses among Albanians, Bosniaks, Gorani and Turks living in south Kosovo. J. Ethnobiol. Ethnomed. 2015, 11. [Google Scholar] [CrossRef] [Green Version]
- Guarrera, P.M. Traditional phytotherapy in Central Italy (Marche, Abruzzo, and Latium). Fitoterapia 2005, 76, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Lucchetti, L.; Zitti, S.; Taffetani, F. Ethnobotanical uses in the Ancona district (Marche region, Central Italy). J. Ethnobiol. Ethnomed. 2019, 15, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, P.T.M.; Silva, M.A.F.; Silva, L.; Seca, A.M.L. Ethnobotanical knowledge in sete cidades, azores archipelago: First ethnomedicinal report. Plants 2019, 8, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabherr, G. Biodiversity in the high ranges of the Alps: Ethnobotanical and climate change perspectives. Glob. Environ. Chang. 2009, 19, 167–172. [Google Scholar] [CrossRef]
- Abbet, C.; Mayor, R.; Roguet, D.; Spichiger, R.; Hamburger, M.; Potterat, O. Ethnobotanical survey on wild alpine food plants in Lower and Central Valais (Switzerland). J. Ethnopharmacol. 2014, 151, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Aro, A.A.; Perez, M.O.; Vieira, C.P.; Esquisatto, M.A.M.; Rodrigues, R.A.F.; Gomes, L.; Pimentel, E.R. Effect of Calendula officinalis cream on achilles tendon healing. Anat. Rec. 2015, 298, 428–435. [Google Scholar] [CrossRef]
- Grosu, E.; Ichim, M.C. Turning Meadow Weeds Into Valuable Species for the Romanian Ethnomedicine While Complying with the Environmentally Friendly Farming Requirements of the European Union’s Common Agricultural Policy. Front. Pharmacol. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Zhelev, I.; Dimitrova-Dyulgerova, I. Antioxidant Activity of Some Carduus Species Growing in Bulgaria. Free Radic. Antioxid. 2011, 1, 15–20. [Google Scholar] [CrossRef]
- Street, R.A.; Sidana, J.; Prinsloo, G. Cichorium intybus: Traditional Uses, Phytochemistry, Pharmacology, and Toxicology. Evid. Based Complement. Altern. Med. eCAM 2013, 2013, 579319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satmbekova, D.; Srivedavyasasri, R.; Orazbekov, Y.; Omarova, R.; Datkhayev, U.; Ross, S.A. Chemical and biological studies on Cichorium intybus L. Nat. Prod. Res. 2018, 32, 1343–1347. [Google Scholar] [CrossRef] [PubMed]
- Subiza, J.L.; Subiza, J.L.; Alonso, M.; Hinojosa, M.; Garcia, R.; Jerez, M.; Subiza, E. Allergic conjunctivitis to chamomile tea. Ann. Allergy 1990, 65, 127–132. [Google Scholar] [PubMed]
- Srivastava, J.K.; Gupta, S. Extraction, characterization, stability and biological activity of flavonoids isolated from chamomile flowers. Mol. Cell. Pharmacol. 2009, 1, 138–147. [Google Scholar] [CrossRef]
- Shin, S.; Lee, J.-A.; Kim, M.; Kum, H.; Jung, E.; Park, D. Anti-Glycation Activities of Phenolic Constituents from Silybum marianum (Milk Thistle) Flower in Vitro and on Human Explants. Molecules 2015, 20, 3549–3564. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Takanaka, K.; Tsukada, H.; Miwa, Y.; Taga, T.; Tanaka, S.; Ikeshiro, Y. Sesquiterpene Glucosides From Anti-Leukotriene B4 Release Fraction of Taraxacum officinale. J. Asian Nat. Prod. Res. 2001, 3, 191–197. [Google Scholar] [CrossRef]
- Sahu, N.; Arya, K. Ethnobotanical and ethnopharmacological activites of Artemisia nilagirica, Lyonia ovalifolia, Sarcococca saligna and Taraxacum officinale. J. Chem. Inf. Model. 2017, 8, 4818–4825. [Google Scholar] [CrossRef]
- Park, C.M.; Park, J.Y.; Noh, K.H.; Shin, J.H.; Song, Y.S. Taraxacum officinale Weber extracts inhibit LPS-induced oxidative stress and nitric oxide prodction via the NF-κB modulation in RAW 264,7 cells. J. Ethnopharmacol. 2011, 133, 834–842. [Google Scholar] [CrossRef]
- Azman, N.A.M.; Skowyra, M.; Muhammad, K.; Gallego, M.G.; Almajano, M.P. Evaluation of the antioxidant activity of Betula pendula leaves extract and its effects on model foods. Pharm. Biol. 2017, 55, 912–919. [Google Scholar] [CrossRef]
- Pavlovic, M.; Kovacevic, N.; Tzakou, O.; Couladis, M. The essential oil of Valeriana officinalis L. s.l. growing wild in western serbia. J. Essent. Oil Res. 2004, 16, 397–399. [Google Scholar] [CrossRef]
- Fernandez, A.; Edwin Cock, I. The Therapeutic Properties of Juniperus communis L.: Antioxidant Capacity, Bacterial growth Inhibition, Anticancer Activity and Toxicity. Pharmacogn. J. 2016, 8, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Al-Snafi, A.E. Medical Importance of Juniperus communis—A Review. Indo Am. J. Pharm. Sci. 2018, 5, 1779–1792. [Google Scholar] [CrossRef]
- Cioanca, O.; Hancianu, M.; Mihasan, M.; Hritcu, L. Anti-acetylcholinesterase and Antioxidant Activities of Inhaled Juniper Oil on Amyloid Beta (1–42)-Induced Oxidative Stress in the Rat Hippocampus. Neurochem. Res. 2015, 40, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Raudone, L.; Vilkickyte, G.; Pitkauskaite, L.; Raudonis, R.; Vainoriene, R.; Motiekaityte, V. Antioxidant Activities of Vaccinium vitis-idaea L. Leaves within Cultivars and Their Phenolic Compounds. Molecules 2019, 24, 844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzaee, F.; Hosseini, A.; Jouybari, H.B.; Davoodi, A.; Azadbakht, M. Medicinal, biological and phytochemical properties of Gentiana species. J. Tradit. Complement. Med. 2017, 7, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Rostami, H.; Kazemi, M.; Shafiei, S. Antibacterial activity of Lavandula officinalis and Melissa officinalis against some human pathogenic bacteria. Asian J. Biochem. 2012, 7, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Mori, H.-M.; Kawanami, H.; Kawahata, H.; Aoki, M. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-β in a rat model. BMC Complement. Altern. Med. 2016, 16, 144. [Google Scholar] [CrossRef] [Green Version]
- Miraj, S.; Rafieian-Kopaei, M.; Kiani, S. Melissa officinalis L: A Review Study With an Antioxidant Prospective. J. Evid. Based. Complementary Altern. Med. 2017, 22, 385–394. [Google Scholar] [CrossRef]
- Ahmad, M.; Sultana, S.; Fazl-i-Hadi, S.; ben Hadda, T.; Rashid, S.; Zafar, M.; Khan, M.; Khan, M.P.; Yaseen, G. An Ethnobotanical study of Medicinal Plants in high mountainous region of Chail valley (District Swat Pakistan). J. Ethnobiol. Ethnomed. 2014, 10, 36. [Google Scholar] [CrossRef] [Green Version]
- Teweldemedhin, M.; Gebreyesus, H.; Atsbaha, A.H.; Asgedom, S.W.; Saravanan, M. Bacterial profile of ocular infections: A systematic review. BMC Ophthalmol. 2017, 17, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kerkoub, N.; Panda, S.K.; Yang, M.-R.; Lu, J.-G.; Jiang, Z.-H.; Nasri, H.; Luyten, W. Bioassay-Guided Isolation of Anti-Candida Biofilm Compounds From Methanol Extracts of the Aerial Parts of Salvia officinalis (Annaba, Algeria). Front. Pharmacol. 2018, 9, 1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dall’Acqua, S.; Viola, G.; Giorgetti, M.; Loi, M.C.; Innocenti, G. Two new sesquiterpene lactones from the leaves of Laurus nobilis. Chem. Pharm. Bull. 2006, 54, 1187–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caputo, L.; Nazzaro, F.; Souza, L.; Aliberti, L.; De Martino, L.; Fratianni, F.; Coppola, R.; De Feo, V. Laurus nobilis: Composition of Essential Oil and Its Biological Activities. Molecules 2017, 22, 930. [Google Scholar] [CrossRef] [PubMed]
- Jeambey, Z.; Johns, T.; Talhouk, S.; Batal, M. Perceived health and medicinal properties of six species of wild edible plants in north-east Lebanon. Public Health Nutr. 2009, 12, 1902–1911. [Google Scholar] [CrossRef] [Green Version]
- Neha, S.; Ritu, R.; Manju, K.; Birendra, K. A review on biological activities of hydrazone derivatives. Int. J. Pharm. Clin. Res. 2016, 8, 162–166. [Google Scholar]
- Ouelbani, R.; Bensari, S.; Mouas, T.N.; Khelifi, D. Ethnobotanical investigations on plants used in folk medicine in the regions of Constantine and Mila (North-East of Algeria). J. Ethnopharmacol. 2016, 194, 196–218. [Google Scholar] [CrossRef]
- Pranskuniene, Z.; Ratkeviciute, K.; Simaitiene, Z.; Pranskunas, A.; Bernatoniene, J. Ethnobotanical Study of Cultivated Plants in Kaišiadorys District, Lithuania: Possible Trends for New Herbal Based Medicines. Evid. Based Complement. Altern. Med. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Tsioutsiou, E.E.; Giordani, P.; Hanlidou, E.; Biagi, M.; De Feo, V.; Cornara, L. Ethnobotanical Study of Medicinal Plants Used in Central Macedonia, Greece. Evid. Based Complement. Altern. Med. 2019, 2019. [Google Scholar] [CrossRef]
- Vogl, S.; Picker, P.; Mihaly-Bison, J.; Fakhrudin, N.; Atanasov, A.G.; Heiss, E.H.; Wawrosch, C.; Reznicek, G.; Dirsch, V.M.; Saukel, J.; et al. Ethnopharmacological in vitro studies on Austria’s folk medicine—An unexplored lore in vitro anti-inflammatory activities of 71 Austrian traditional herbal drugs. J. Ethnopharmacol. 2013, 149, 750–771. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Lee, K.; Lee, M.-H.H.; Kim, S.-H.H.; Ham, I.-H.H.; Choi, H.-Y.Y. Inhibitory effects of Chelidonium majus extract on atopic dermatitis-like skin lesions in NC/Nga mice. J. Ethnopharmacol. 2011, 138, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Baldan, V.; Sut, S.; Faggian, M.; Dalla Gassa, E.; Ferrari, S.; De Nadai, G.; Francescato, S.; Baratto, G.; Dall’Acqua, S. Larix decidua Bark as a Source of Phytoconstituents: An LC-MS Study. Molecules 2017, 22, 1974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dar, P.A.; Sofi, G.; Jafri, M.A. Polypodium vulgare Linn. a versatile herbal medicine: A review. Int. J. Pharm. Sci. Res. 2012, 3, 1616–1620. [Google Scholar] [CrossRef]
- Naz, S.B.; Chaudhry, M.A.; Rahaman, S.U. Dual receptors blocked mechanism arbitrates smooth muscles relaxant effect of Polypodium vulgare. Bangladesh J. Pharmacol. 2016, 11, 414. [Google Scholar] [CrossRef]
- Khan, A.; Siddiqui, A.; Jafri, M.A.; Asif, M. Ethnopharmacological Studies of Polypodium vulgare Linn: A Comprehensive Review. J. Drug Deliv. Ther. 2018, 8, 73–76. [Google Scholar] [CrossRef] [Green Version]
- Ghedira, K.; Goetz, P.; Le Jeune, R. Alchemilla vulgaris L.: Alchémille (Rosaceae). Phytothérapie 2012, 10, 263–266. [Google Scholar] [CrossRef]
- Caliskan, O. Mediterranean Hawthorn Fruit (Crataegus) Species and Potential Usage. In The Mediterranean Diet: An Evidence-Based Approach; Elsevier: Amsterdam, The Netherlands, 2015; pp. 621–628. [Google Scholar]
- Djeddi, S.; Boutaleb, H. Evaluation of antioxidative and antibacterial potentials of Crataegus monogyna Jacq. from Evaluation of antioxidative and antibacterial potentials of Crataegus monogyna Jacq. from Mahouna mountain ( Algeria ). J. Adv. Sci. Appl. Eng. 2015, 1, 60–63. [Google Scholar]
- Malanga, G.A.; Yan, N.; Stark, J. Mechanisms and efficacy of heat and cold therapies for musculoskeletal injury. Postgrad. Med. 2015, 127, 57–65. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| Species | Preparation | Traditional Recipe |
|---|---|---|
| Arnica montana L. subsp. montana Arnica | Inflorescences exudate | Put the inflorescences in an empty glass flagon. Keep it under the summer sun. Turn the flagon upside down in order to help the exudate to leak from the inflorescences, regularly gathering the liquid. |
| Calendula officinalis L. Calendula | Macerated oil | Fill a glass jar to the brim with inflorescences. Cover the flowers with almond oil. Keep the jar under the sun for 20–30 days. Filter the macerated oil. |
| Hypericum perforatum L. Hypericum | Macerated oil | Fill a glass jar to the brim with inflorescences collected in June–July. Cover the flowers with almond, olive, or sunflower oil. Keep the jar under the summer sun. Filter the macerated oil in September. |
| Larix decidua Mill. Larch | Ointment | Mix 1 Kg of spruce and larch resin with 100g of butter, olive oil, and bee wax until it reaches a smooth texture. |
| Picea abies (L.) H.Karst Spruce | ||
| Pinus mugo Turra Mountain pine | Pinecones syrup | Put green pinecones collected in June in a glass jar until they reach the brim. Cover the pinecone completely with white sugar. Keep the jar under the summer sun for 60 days, shaking it at intervals. Filter the syrup. |
| Taraxacum spp. Dandelion | Inflorescences syrup | Put 100 flower heads in a glass jar and cover them with 100 g of white sugar. Keep the jar under the summer sun for 30–40 days, until the sugar melts. Filter the syrup. |
| Category of Use (Pathologies) | n. Species per Category | n. Citations per Category | ICF |
|---|---|---|---|
| Digestive tract disorders | 28 | 297 | 0.91 |
| Respiratory tract infections | 20 | 348 | 0.95 |
| General condition | 20 | 103 | 0.81 |
| Urinary tract disorders | 20 | 73 | 0.74 |
| Musculoskeletal system disorders and traumas | 16 | 410 | 0.96 |
| Skin diseases and traumas | 14 | 140 | 0.91 |
| Nervous system disorders | 12 | 97 | 0.89 |
| Circulatory system disorders | 12 | 37 | 0.69 |
| Gynecological disorders, obstetric and puerperal problems | 8 | 21 | 0.65 |
| Ophthalmic ailments | 7 | 36 | 0.83 |
| Other | 7 | 19 | 0.67 |
| Oropharyngeal cavity affections | 6 | 25 | 0.79 |
| Early infancy ailments | 1 | 6 | 1.00 |
| Afflictions of the ear | 1 | 1 |
| Comparisons | Uses Reported in Both Groups | Uses Reported in One Group Only (Group 1/Group 2) | Jaccard Index | Reference | Specific Uses in Valmalenco | |
|---|---|---|---|---|---|---|
| VM | VV | 19 | 162/66 | 0.09 | [19] | 162 |
| VM | VSG | 41 | 140/133 | 0.18 | [13] | 140 |
| VM | VF | 69 | 112/209 | 0.27 | [3] | 112 |
| VM | LS | 89 | 92/539 | 0.16 | [15] | 92 |
| VM | WA1 | 17 | 164/53 | 0.08 | [22] | 164 |
| VM | WA2 | 22 | 159/130 | 0.08 | [2] | 159 |
| VM | NW | 24 | 157/198 | 0.07 | [14] | 157 |
| VM | VAR | 15 | 166/38 | 0.08 | [18] | 166 |
| VM | TR | 48 | 133/181 | 0.18 | [17] | 133 |
| Average | 0.13 | Average | 142.78 | |||
| Standard deviation | 26.11 | |||||
| Latin Name | Traditional Uses in Valmalenco | Type of Preparation in Literature | Reference | |||
|---|---|---|---|---|---|---|
| Part of the Plant and Preparation | Field of Use | Category of Use | Detailed Use | |||
| Achillea millefolium L. | Epigeal part (Whole) (compresses with infusion) | Med | Skin diseases and traumas | Anti-inflammatory, disinfectant and wound healing, Emollient, soothing | Hot water extract with hemostyptic activity in vitro | [25] |
| Aqueous extract with anti-inflammatory activity in vitro | [24] | |||||
| Matricaria chamomilla L. | Flowers/inflorescences/flowering tops (infusion) | Med | - | Anti-oxidant | Aqueous extract with anti-oxidant activity in vitro | [108] |
| Flowers/inflorescences/flowering tops (infusion) | Med | Nervous system disorders | Sedative hypnotic, promotes sleep | Lyophilized aqueous extract with activity on CNS | ||
| Taraxacum spp. | Flowers/inflorescences/flowering tops (infusion) | Med | Urinary tract disorders | Kidney stones Cystitis and other inflammation of the urinary tract Urinary tract depurative Diuretic | Aqueous roots extract with diuretic activity in rats | [167] |
| Leaves (infusion or decoction) | ||||||
| Epigeal part (Whole) (infusion) | ||||||
| Underground organs (roots/bulbs/tubers/rhizomes) (infusion or decoction) | ||||||
| Leaves (infusion or decoction) | Med | - | Anti-inflammatory | Aqueous leaves extract with anti-inflammatory activity in vivo | ||
| Gentiana lutea L. subsp. lutea | Underground organs (roots/bulbs/tubers/rhizomes) (decoction, grappa) | Med | Digestive tract disorders | Digestive Stomach anti-inflammatory Liver anti-inflammatory Stomachache Vermifuge | EtOH roots extract with choleretic activity (not the Aqueous nor the MeOH extracts) | WHO Monograph—Radix Gentianae Luinfusione [29] |
| Hypericum perforatum L. | Flowers/inflorescences/flowering tops (macerated oil) | Med | Skin diseases and traumas | Sunburns, burns, frostbites, redness and rash Anti-inflammatory, disinfectant and wound healing Psoriasis Insect bites | Different types of macerated oils active on burns and wounds | [72] |
| 21 macerated oil samples of H. (homemade or commercial) analyzed. Pseudohypericin and hypericin in all samples. Hyperforin in 4 samples. | ||||||
| Flowers/inflorescences/flowering tops (macerated oil) | Med | Musculoskeletal system disorders and traumas | Contusions Sprains and dislocations Articular pain and inflammations Muscle inflammations and pain | |||
| Origanum vulgare L. | Epigeal part (Whole) (infusion/compresses with infusion) | Med | - | Antioxidant activity | Antioxidant activity of hot and cold water extracts | [121] |
| Rosmarinus officinalis L. | Epigeal part (Whole) (infusion) | Med | Digestive tract disorders | Liver depurative | Choleretic and Hepatoprotective activity of aqueous extracts in rats | [141] |
| Hepatoprotective and antioxidant activity of aqueous extract in rats | [144] | |||||
| Thymus spp. | Epigeal part (Whole) (infusion) | Med | Circulatory system disorders | Blood depurative | Hepatoprotective and antioxidant activity of aqueous extract in rats | [144] |
| Flowers/inflorescences/flowering tops (infusion) | Med | Respiratory tract infections | Balsamic Cough Colds and flu symptoms Expectorant, decongestant, emollient | Antimicrobial activity of aqueous extract | [170,199] | |
| Leaves (infusion) | ||||||
| Epigeal part (Whole) (infusion) | ||||||
| Laurus nobilis L. | Fruits/infructescences/accessory fruits (infusion) | Med | Digestive tract disorders | Antiacid, gastritis, acid reflux Carminative Abdominal pain | Decoction in water of fruits with gastroprotective activity | [90] |
| Linum usitatissimum L. | Seeds (boiled or left in water overnight, then drunk) | Med | Digestive tract disorders | Laxative, intestinal motility | Maceration in how water to extract mucilages | [95] |
| Malva sylvestris L. | Leaves (infusion, compresses/baths with infusion) | Med | - | Analgesic | Aqueous extract with analgesic activity in rats (intraperitoneal administration) | [100] |
| Leaves (infusion) | Med | Urinary tract disorders | Depurative | Nephroprotecive activity of aqueous extract of leaves and flowers | [106] | |
| Epilobium spp. | Leaves (infusion) | Med | General condition | Anti-inflammatory | Aqueous extracts with activity on COX-1 and COX-2 | [58] |
| Aqueous extracts with anti-inflammatory activity in vitro | [59] | |||||
| Chelidonium majus L. | Latex or sap (applied raw) | Med | Skin diseases and traumas | Skin tags and warts | Case report: 4-years child with warts treated with raw latex | [55] |
| Cetraria islandica (L.) Ach. subsp. islandica | Epigeal part (Whole) (infusion) | Med | Respiratory tract infections | Expectorant, decongestant, emollientSorethroat and hoarseness | Anti-inflammatory activity of aqueous extract in vitro | [51] |
| Passiflora spp. | Epigeal part (Whole) (infusion) | Med | Nervous system disorders | Promotes sleep | Effect of infusion on Subjective sleep quality | [126] |
| Plantago major L. | Leaves (washing with infusion) | Med | Oropharyngeal cavity affections | Gingivitis, toothache, mouth ulcers and abscesses | Anti-inflammatory activity of extract in deionized water in vivo | [129] |
| Rosa canina L. | Fruits/infructescences/accessory fruits (infusion) | Med | Respiratory tract infections | Decongestant Sorethroat | Different aqueous extracts of rose hips with anti-inflammatory activity | [140] |
| Urtica dioica L. | Leaves (applied as a poultice) | Med | Musculoskeletal system disorders and traumas | Rheumatism, Contusions, Hematoma | Leaves applied on thumb against osteoarthritis showed anti-inflammatory activity | [176] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bottoni, M.; Milani, F.; Colombo, L.; Nallio, K.; Colombo, P.S.; Giuliani, C.; Bruschi, P.; Fico, G. Using Medicinal Plants in Valmalenco (Italian Alps): From Tradition to Scientific Approaches. Molecules 2020, 25, 4144. https://doi.org/10.3390/molecules25184144
Bottoni M, Milani F, Colombo L, Nallio K, Colombo PS, Giuliani C, Bruschi P, Fico G. Using Medicinal Plants in Valmalenco (Italian Alps): From Tradition to Scientific Approaches. Molecules. 2020; 25(18):4144. https://doi.org/10.3390/molecules25184144
Chicago/Turabian StyleBottoni, Martina, Fabrizia Milani, Lorenzo Colombo, Kevin Nallio, Paola Sira Colombo, Claudia Giuliani, Piero Bruschi, and Gelsomina Fico. 2020. "Using Medicinal Plants in Valmalenco (Italian Alps): From Tradition to Scientific Approaches" Molecules 25, no. 18: 4144. https://doi.org/10.3390/molecules25184144
APA StyleBottoni, M., Milani, F., Colombo, L., Nallio, K., Colombo, P. S., Giuliani, C., Bruschi, P., & Fico, G. (2020). Using Medicinal Plants in Valmalenco (Italian Alps): From Tradition to Scientific Approaches. Molecules, 25(18), 4144. https://doi.org/10.3390/molecules25184144