Influence of Membrane Phase on the Optical Properties of DPH
Abstract
:1. Introduction
2. Results and Discussion
2.1. QM and MD Dihedral Analyses
2.2. One Photon Absorption
2.3. Non-Linear Optical Properties
2.3.1. Two Photon Absorption
2.3.2. Hyper-Rayleigh Scattering
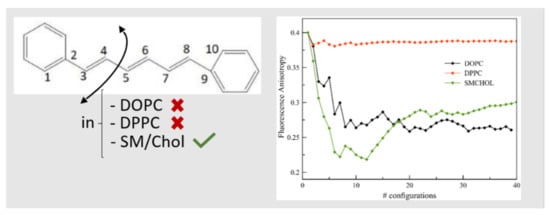
2.4. Fluorescence Properties
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Calculation of the Conformational Abundance


| Conformer | ΔE |
|---|---|
| tct | 2.02 |
| ctt | 3.70 |
| cct | 5.85 |
| ctc | 8.38 |
| ccc | 10.86 |



Appendix B
| DOPC | Wavelength | Λ | Transition (%) |
|---|---|---|---|
| S1 | 336.8 (1.94) | 0.83 | H -> L (100 %) |
| S2 | 264.7 (0.01) | 0.58 | H -> L + 1 (90 %) |
| S3 | 257.0 (0.01) | 0.46 | H -> L + 2 (26 %) |
| H − 2 -> L (23 %) | |||
| H − 1 -> L + 3 (21 %) | |||
| DPPC | Wavelength | Λ | Transition (%) |
| S1 | 345.6 (1.91) | 0.80 | H -> L (100 %) |
| S2 | 276.5 (0.02) | 0.56 | H -> L + 1 (85 %) |
| S3 | 270.3 (0.01) | 0.47 | H − 2 -> L (35 %) |
| H -> L + 3 (22 %) | |||
| SMCHOL | Wavelength | Λ | Transition (%) |
| S1 | 337.2 (1.65) | 0.79 | H -> L (100 %) |
| S2 | 256.1 (0.03) | 0.55 | H -> L + 1 (90 %) |
| S3 | 266.7 (0.02) | 0.49 | H − 1 -> L (35 %) |
| H − 2 -> L (15 %) | |||
| H − 1 -> L + 3 (15 %) |

Appendix C
| TPA DOPC | Aver | STD (%) |
|---|---|---|
| S1 | 8.87 | 1.78 (20) |
| S2 | 131.3 | 33.62 (26) |
| S3 | 27.6 | 3.45 (12) |
| TPA DPPC | Aver | STD (%) |
| S1 | 61.1 | 8.94 (15) |
| S2 | 51.6 | 10.26 (20) |
| S3 | 48.8 | 11.74 (24) |
| TPA SM/Chol | Aver | STD (%) |
| S1 | 43.8 | 13.15 (30) |
| S2 | 51.6 | 9.49 (18) |
| S3 | 48.8 | 10.41 (21) |
| HRS static. | Aver | STD (%) |
|---|---|---|
| DOPC | 5.33 | 3.81 (70) |
| DPPC | 17.7 | 5.85 (33) |
| SM/Chol | 7.07 | 5.35 (75) |
| HRS 810 nm | Aver | STD (%) |
| DOPC | 35.7 | 5.27 (15) |
| DPPC | 128.7 | 18.5 (14) |
| SM/Chol | 62.4 | 11.3 (18) |
References
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Enkavi, G.; Javanainen, M.; Kulig, W.; Róg, T.; Vattulainen, I. Multiscale Simulations of Biological Membranes: The Challenge To Understand Biological Phenomena in a Living Substance. Chem. Rev. 2019, 119, 5607–5774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Risselada, H.J.; Grubmueller, H. How SNARE molecules mediate membrane fusion: Recent insights from molecular simulations. Curr. Opin. Struct. Biol. 2012, 22, 187–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; So, L.; Spector, A. Membrane Cholesterol and Phospholipid in Consecutive Concentric Sections of Human Lenses. J. Lipid Res. 1985, 26, 600–609. [Google Scholar] [PubMed]
- Deliconstantinos, G. Physiological-Aspects of Membrane Lipid Fluidity in Malignancy. Anticancer Res. 1987, 7, 1011–1022. [Google Scholar] [PubMed]
- Daefler, S.; Krueger, G.R.; Modder, B.; Deliconstantinos, G. Cell membrane fluidity in chronic lymphocytic leukemia (CLL) lymphocytes and its relation to membrane receptor expression. J. Exp. Pathol. 1987, 3, 147–154. [Google Scholar]
- Coughlin, M.F.; Bielenberg, D.R.; Lenormand, G.; Marinkovic, M.; Waghorne, C.G.; Zetter, B.R.; Fredberg, J.J. Cytoskeletal stiffness, friction, and fluidity of cancer cell lines with different metastatic potential. Clin. Exp. Metastasis 2013, 30, 237–250. [Google Scholar] [CrossRef] [Green Version]
- Braig, S.; Schmidt, B.U.S.; Stoiber, K.; Haendel, C.; Moehn, T.; Werz, O.; Mueller, R.; Zahler, S.; Koeberle, A.; Kaes, J.A.; et al. Pharmacological targeting of membrane rigidity: implications on cancer cell migration and invasion. New J. Phys. 2015, 17, 083007. [Google Scholar] [CrossRef]
- Haendel, C.; Schmidt, B.U.S.; Schiller, J.; Dietrich, U.; Moehn, T.; Kiessling, T.R.; Pawlizak, S.; Fritsch, A.W.; Horn, L.-C.; Briest, S.; et al. Cell membrane softening in human breast and cervical cancer cells. New J. Phys. 2015, 17, 083008. [Google Scholar] [CrossRef]
- Erazo-Oliveras, A.; Fuentes, N.R.; Wright, R.C.; Chapkin, R.S. Functional link between plasma membrane spatiotemporal dynamics, cancer biology, and dietary membrane-altering agents. Cancer Metastasis Rev. 2018, 37, 519–544. [Google Scholar] [CrossRef]
- Brown, D.A.; London, E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 1998, 14, 111–136. [Google Scholar] [CrossRef] [PubMed]
- Sherbet, G. Membrane Fluidity and Cancer Metastasis. Exp. Cell Biol. 1989, 57, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Bloom, M. The physics of soft, natural materials. Phys. Can. 1992, 48, 7–16. [Google Scholar]
- Iwagaki, H.; Marutaka, M.; Nezu, M.; Suguri, T.; Tanaka, N.; Orita, K. Cell-Membrane Fluidity in K562 Cells and Its Relation to Receptor Expression. Res. Commun. Mol. Pathol. Pharmacol. 1994, 85, 141–149. [Google Scholar] [PubMed]
- Taraboletti, G.; Perin, L.; Bottazzi, B.; Mantovani, A.; Giavazzi, R.; Salmona, M. Membrane Fluidity Affects Tumor-Cell Motility, Invasion and Lung-Colonizing Potential. Int. J. Cancer 1989, 44, 707–713. [Google Scholar] [CrossRef]
- Nakazawa, I.; Iwaizumi, M. A Role of the Cancer Cell-Membrane Fluidity in the Cancer Metastases. Tohoku J. Exp. Med. 1989, 157, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Zalba, S.; ten Hagen, T.L.M. Cell membrane modulation as adjuvant in cancer therapy. Cancer Treat. Rev. 2017, 52, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Islam, S.R.; Manna, S.K. Lipidomic Analysis of Cancer Cell and Tumor Tissues. In Cancer Metabolism; Haznadar, M., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; Volume 1928, pp. 175–204. ISBN 978-1-4939-9026-9. [Google Scholar]
- Gasecka, A.; Han, T.-J.; Favard, C.; Cho, B.R.; Brasselet, S. Quantitative Imaging of Molecular Order in Lipid Membranes Using Two-Photon Fluorescence Polarimetry. Biophys. J. 2009, 97, 2854–2862. [Google Scholar] [CrossRef]
- Gaus, K.; Zech, T.; Harder, T. Visualizing membrane microdomains by Laurdan 2-photon microscopy (Review). Mol. Membr. Biol. 2006, 23, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Margineanu, A.; Hotta, J.; Van der Auweraer, M.; Ameloot, M.; Stefan, A.; Beljonne, D.; Engelborghs, Y.; Herrmann, A.; Müllen, K.; De Schryver, F.C.; et al. Visualization of Membrane Rafts Using a Perylene Monoimide Derivative and Fluorescence Lifetime Imaging. Biophys. J. 2007, 93, 2877–2891. [Google Scholar] [CrossRef] [Green Version]
- Parmryd, I.; Önfelt, B. Consequences of membrane topography. FEBS J. 2013, 280, 2775–2784. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Sampaio, J.L. Membrane Organization and Lipid Rafts. Cold Spring Harb. Perspect. Biol. 2011, 3, a004697. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Vaz, W.L.C. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 2004, 33, 269–295. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S.; Tricerri, M.A.; Gunther, G.; Gratton, E. Laurdan generalized polarization: From cuvette to microscope. In Modern Research and Educational Topics in Microscopy; Formatex Research Center: Badajoz, Spain, 2007; pp. 1007–1014. [Google Scholar]
- Okur, H.I.; Tarun, O.B.; Roke, S. Chemistry of Lipid Membranes from Models to Living Systems: A Perspective of Hydration, Surface Potential, Curvature, Confinement and Heterogeneity. J. Am. Chem. Soc. 2019, 141, 12168–12181. [Google Scholar] [CrossRef]
- Filipe, H.A.L.; Moreno, M.J.; Loura, L.M.S. The Secret Lives of Fluorescent Membrane Probes as Revealed by Molecular Dynamics Simulations. Molecules 2020, 25, 3424. [Google Scholar] [CrossRef] [PubMed]
- Murugan, N.A.; Apostolov, R.; Rinkevicius, Z.; Kongsted, J.; Lindahl, E.; Agren, H. Association Dynamics and Linear and Nonlinear Optical Properties of an N-Acetylaladanamide Probe in a POPC Membrane. J. Am. Chem. Soc. 2013, 135, 13590–13597. [Google Scholar] [CrossRef]
- Osella, S.; Murugan, N.A.; Jena, N.K.; Knippenberg, S. Investigation into Biological Environments through (Non)linear Optics: A Multiscale Study of Laurdan Derivatives. J. Chem. Theory Comput. 2016, 12, 6169–6181. [Google Scholar] [CrossRef]
- Osella, S.; Knippenberg, S. Triggering On/Off States of Photoswitchable Probes in Biological Environments. J. Am. Chem. Soc. 2017, 4418–4428. [Google Scholar] [CrossRef]
- Osella, S.; Di Meo, F.; Murugan, N.A.; Fabre, G.; Ameloot, M.; Trouillas, P.; Knippenberg, S. Combining (Non)linear Optical and Fluorescence Analysis of DiD To Enhance Lipid Phase Recognition. J. Chem. Theory Comput. 2018, 14, 5350–5359. [Google Scholar] [CrossRef] [Green Version]
- Paloncýová, M.; Aniander, G.; Larsson, E.; Knippenberg, S. Cyanine dyes with tail length asymmetry enhance photoselection: A multiscale study on DiD probes in a liquid disordered membrane. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 224, 117329. [Google Scholar] [CrossRef]
- Osella, S.; Knippenberg, S. Laurdan as a Molecular Rotor in Biological Environments. ACS Appl. Bio Mater. 2019, 2, 5769–5778. [Google Scholar] [CrossRef]
- Knippenberg, S.; Osella, S. Push/Pull Effect as Driving Force for Different Optical Responses of Azobenzene in a Biological Environment. J. Phys. Chem. C 2020, 124, 8310–8322. [Google Scholar] [CrossRef]
- Allen, M.J.; Tozer, D.J. Helium dimer dispersion forces and correlation potentials in density functional theory. J. Chem. Phys. 2002, 117, 11113–11120. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S. Accurate description of van der Waals complexes by density functional theory including empirical corrections. J. Comput. Chem. 2004, 25, 1463–1473. [Google Scholar] [CrossRef]
- Morini, F.; Knippenberg, S.; Deleuze, M.S.; Hajgato, B. Quantum Chemical Study of Conformational Fingerprints in the Photoelectron Spectra and (e, 2e) Electron Momentum Distributions of n-Hexane. J. Phys. Chem. A 2010, 114, 4400–4417. [Google Scholar] [CrossRef]
- Saltiel, J.; Klima, R.; van de Burgt, L.J.; Wang, S.; Dmitrenko, O. Temperature Dependence of the 1,6-Diphenyl-1,3,5-hexatriene Triplet Lifetime in Solution and Theoretical Evaluation of Triplet Conformer Interconversion. J. Phys. Chem. B 2010, 114, 14480–14486. [Google Scholar] [CrossRef]
- Paloncyova, M.; Ameloot, M.; Knippenberg, S. Orientational distribution of DPH in lipid membranes: A comparison of molecular dynamics calculations and experimental time-resolved anisotropy experiments. Phys. Chem. Chem. Phys. 2019, 21, 7594–7604. [Google Scholar] [CrossRef]
- Peach, M.J.G.; Benfield, P.; Helgaker, T.; Tozer, D.J. Excitation energies in density functional theory: An evaluation and a diagnostic test. J. Chem. Phys. 2008, 128, 044118. [Google Scholar] [CrossRef]
- Knippenberg, S.; Fabre, G.; Osella, S.; Di Meo, F.; Paloncyova, M.; Ameloot, M.; Trouillas, P. Atomistic Picture of Fluorescent Probes with Hydrocarbon Tails in Lipid Bilayer Membranes: An Investigation of Selective Affinities and Fluorescent Anisotropies in Different Environmental Phases. Langmuir 2018, 34, 9072–9084. [Google Scholar] [CrossRef]
- Falkovich, S.G.; Martinez-Seara, H.; Nesterenko, A.M.; Vattulainen, I.; Gurtovenko, A.A. What Can We Learn about Cholesterol’s Transmembrane Distribution Based on Cholesterol-Induced Changes in Membrane Dipole Potential? J. Phys. Chem. Lett. 2016, 7, 4585–4590. [Google Scholar] [CrossRef]
- Gurtovenko, A.A.; Vattulainen, I. Calculation of the electrostatic potential of lipid bilayers from molecular dynamics simulations: Methodological issues. J. Chem. Phys. 2009, 130, 215107. [Google Scholar] [CrossRef] [PubMed]
- Slenders, E.; Seneca, S.; Pramanik, S.K.; Smisdom, N.; Adriaensens, P.; vandeVen, M.; Ethirajan, A.; Ameloot, M. Dynamics of the phospholipid shell of microbubbles: a fluorescence photoselection and spectral phasor approach. Chem. Commun. 2018, 54, 4854–4857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacalum, M.; Wang, L.; Boodts, S.; Yuan, P.; Leen, V.; Smisdom, N.; Fron, E.; Knippenberg, S.; Fabre, G.; Trouillas, P.; et al. A Blue-Light-Emitting BODIPY Probe for Lipid Membranes. Langmuir 2016, 32, 3495–3505. [Google Scholar] [CrossRef]
- Campbell, K.R.; Chaudhary, R.; Handel, J.M.; Patankar, M.S.; Campagnola, P.J. Polarization-resolved second harmonic generation imaging of human ovarian cancer. J. Biomed. Opt. 2018, 23, 066501. [Google Scholar] [CrossRef] [Green Version]
- Cox, G.; Moreno, N.; Feijo, J. Second-harmonic imaging of plant polysaccharides. J. Biomed. Opt. 2005, 10, 024013. [Google Scholar] [CrossRef]
- Slenders, E.; Bové, H.; Urbain, M.; Mugnier, Y.; Sonay, A.Y.; Pantazis, P.; Bonacina, L.; Vanden Berghe, P.; vandeVen, M.; Ameloot, M. Image Correlation Spectroscopy with Second Harmonic Generating Nanoparticles in Suspension and in Cells. J. Phys. Chem. Lett. 2018, 9, 6112–6118. [Google Scholar] [CrossRef] [Green Version]
- Diaspro, A. Optical Fluorescence Microscopy: From the Spectral to the Nano Dimension; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010; ISBN 978-3-642-15175-0. [Google Scholar]
- Bouquiaux, C.; Tonnele, C.; Castet, F.; Champagne, B. Second-Order Nonlinear Optical Properties of an Amphiphilic Dye Embedded in a Lipid Bilayer. A Combined Molecular Dynamics-Quantum Chemistry Study. J. Phys. Chem. B 2020, 124, 2101–2109. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Goto, Y.; Suga, K.; Nyholm, T.K.M.; Slotte, J.P.; Umakoshi, H. Solvatochromic Modeling of Laurdan for Multiple Polarity Analysis of Dihydrosphingomyelin Bilayer. Biophys. J. 2019, 116, 874–883. [Google Scholar] [CrossRef] [Green Version]
- Yefimova, S.L.; Tkacheva, T.N.; Kasian, N.A. Study of the Combined Effect of Ibuprofen and Cholesterol on the Microviscosity and Ordering of Model Lipid Membranes by Timeresolved Measurement of Fluorescence Anisotropy Decay. J. Appl. Spectrosc. 2017, 84, 284–290. [Google Scholar] [CrossRef]
- Mize, H.E.; Blanchard, G.J. Interface-mediation of lipid bilayer organization and dynamics. Phys. Chem. Chem. Phys. 2016, 18, 16977–16985. [Google Scholar] [CrossRef] [Green Version]
- Setiawan, I.; Blanchard, G.J. Structural Disruption of Phospholipid Bilayers over a Range of Length Scales by n-Butanol. J. Phys. Chem. B 2014, 118, 3085–3093. [Google Scholar] [CrossRef] [PubMed]
- Swain, J.; Borkar, S.R.; Aidhen, I.S.; Mishra, A.K. A molecular level understanding of interaction between FTY720 (Fingolimod hydrochloride) and DMPC multilamellar vesicles. RSC Adv. 2014, 4, 17347–17353. [Google Scholar] [CrossRef]
- Vequi-Suplicy, C.C.; Lamy, M.T.; Marquezin, C.A. The New Fluorescent Membrane Probe Ahba: A Comparative Study with the Largely Used Laurdan. J. Fluoresc. 2013, 23, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Montaldi, L.R.; Berardi, M.; Souza, E.S.; Juliano, L.; Ito, A.S. End-to-end Distance Distribution in Fluorescent Derivatives of Bradykinin in Interaction with Lipid Vesicles. J. Fluoresc. 2012, 22, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Dunning, T. Gaussian-Basis Sets for Use in Correlated Molecular Calculations .1. the Atoms Boron Through Neon and Hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Shao, Y.; Gan, Z.; Epifanovsky, E.; Gilbert, A.T.B.; Wormit, M.; Kussmann, J.; Lange, A.W.; Behn, A.; Deng, J.; Feng, X.; et al. Advances in molecular quantum chemistry contained in the Q-Chem 4 program package. Mol. Phys. 2015, 113, 184–215. [Google Scholar] [CrossRef] [Green Version]
- Osella, S.; Smisdom, N.; Ameloot, M.; Knippenberg, S. Conformational Changes as Driving Force for Phase Recognition: The Case of Laurdan. Langmuir 2019, 35, 11471–11481. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Chiu, S.-W.; Pandit, S.A.; Scott, H.L.; Jakobsson, E. An Improved United Atom Force Field for Simulation of Mixed Lipid Bilayers. J. Phys. Chem. B 2009, 113, 2748–2763. [Google Scholar] [CrossRef]
- Pandit, S.A.; Chiu, S.-W.; Jakobsson, E.; Grama, A.; Scott, H.L. Cholesterol packing around lipids with saturated and unsaturated chains: a simulation study. Langmuir 2008, 24, 6858–6865. [Google Scholar] [CrossRef] [Green Version]
- Pandit, S.A.; Chiu, S.-W.; Jakobsson, E.; Grama, A.; Scott, H.L. Cholesterol surrogates: a comparison of cholesterol and 16:0 ceramide in POPC bilayers. Biophys. J. 2007, 92, 920–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Open Babel Package, version 2.3.1. Available online: http://openbabel.org (accessed on 17 July 2020).
- Aidas, K.; Angeli, C.; Bak, K.L.; Bakken, V.; Bast, R.; Boman, L.; Christiansen, O.; Cimiraglia, R.; Coriani, S.; Dahle, P.; et al. The Dalton quantum chemistry program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2014, 4, 269–284. [Google Scholar] [CrossRef]
- Dalton, a Molecular Electronic Structure Program, Release Dalton2016 (2015). Available online: http://daltonprogram.org (accessed on 17 July 2020).
- Herzberg, G. Molecular Spectra and Molecular Structure I. Spectra of Diatomic Molecules; Krieger: Malabar, Florida, 1989. [Google Scholar]
- Sundholm, D.; Rizzo, A.; Jorgensen, P. Multiconfiguration Self-Consistent-Field Quadratic Response Calculations. J. Chem. Phys. 1994, 101, 4931–4935. [Google Scholar] [CrossRef]
- Hu, Z.; Autschbach, J.; Jensen, L. Simulation of resonance hyper-Rayleigh scattering of molecules and metal clusters using a time-dependent density functional theory approach. J. Chem. Phys. 2014, 141, 124305. [Google Scholar] [CrossRef] [PubMed]
- Glauber, R.J.; Lewenstein, M. Quantum optics of dielectric media. Phys. Rev. A 1991, 43, 467–491. [Google Scholar] [CrossRef]
- Dols-Perez, A.; Gramse, G.; Calo, A.; Gomila, G.; Fumagalli, L. Nanoscale electric polarizability of ultrathin biolayers on insulating substrates by electrostatic force microscopy. Nanoscale 2015, 7, 18327–18336. [Google Scholar] [CrossRef]
- Gramse, G.; Dols-Perez, A.; Edwards, M.A.; Fumagalli, L.; Gomila, G. Nanoscale Measurement of the Dielectric Constant of Supported Lipid Bilayers in Aqueous Solutions with Electrostatic Force Microscopy. Biophys. J. 2013, 104, 1257–1262. [Google Scholar] [CrossRef] [Green Version]
- Devanathan, S.; Salamon, Z.; Lindblom, G.; Grobner, G.; Tollin, G. Effects of sphingomyelin, cholesterol and zinc ions on the binding, insertion and aggregation of the amyloid A beta(1-40) peptide in solid-supported lipid bilayers. FEBS J. 2006, 273, 1389–1402. [Google Scholar] [CrossRef]
- Kooyman, R.; Vos, M.; Levine, Y. Determination of Orientational Order Parameters in Oriented Lipid-Membrane Systems by Angle-Resolved Fluorescence Depolarization Experiments. Chem. Phys. 1983, 81, 461–472. [Google Scholar] [CrossRef]
- Zannoni, C.; Arcioni, A.; Cavatorta, P. Fluorescence Depolarization in Liquid-Crystals and Membrane Bilayers. Chem. Phys. Lipids 1983, 32, 179–250. [Google Scholar] [CrossRef]
- Ameloot, M.; vandeVen, M.; Acuña, A.U.; Valeur, B. Fluorescence anisotropy measurements in solution: Methods and reference materials (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 589–608. [Google Scholar] [CrossRef]
- Cusati, T.; Granucci, G.; Persico, M. Photodynamics and Time-Resolved Fluorescence of Azobenzene in Solution: A Mixed Quantum-Classical Simulation. J. Am. Chem. Soc. 2011, 133, 5109–5123. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.R.; Knippenberg, S.; Hajgato, B.; Francois, J.-P.; Deng, J.K.; Deleuze, M.S. Imaging momentum orbital densities of conformationally versatile molecules: A benchmark theoretical study of the molecular and electronic structures of dimethoxymethane. J. Phys. Chem. A 2007, 111, 5879–5897. [Google Scholar] [CrossRef]
- Martin, J.M.L. Basis set convergence and performance of density functional theory including exact exchange contributions for geometries and harmonic frequencies. Mol. Phys. 1995, 86, 1437–1450. [Google Scholar] [CrossRef]
- Martin, J.; Elyazal, J.; Francois, J. Structure and Vibrational-Spectra of Carbon Clusters C-N (n=2-10, 12, 14, 16, 18) Using Density-Functional Theory Including Exact Exchange Contributions. Chem. Phys. Lett. 1995, 242, 570–579. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osella, S.; Paloncýová, M.; Sahi, M.; Knippenberg, S. Influence of Membrane Phase on the Optical Properties of DPH. Molecules 2020, 25, 4264. https://doi.org/10.3390/molecules25184264
Osella S, Paloncýová M, Sahi M, Knippenberg S. Influence of Membrane Phase on the Optical Properties of DPH. Molecules. 2020; 25(18):4264. https://doi.org/10.3390/molecules25184264
Chicago/Turabian StyleOsella, Silvio, Markéta Paloncýová, Maryam Sahi, and Stefan Knippenberg. 2020. "Influence of Membrane Phase on the Optical Properties of DPH" Molecules 25, no. 18: 4264. https://doi.org/10.3390/molecules25184264
APA StyleOsella, S., Paloncýová, M., Sahi, M., & Knippenberg, S. (2020). Influence of Membrane Phase on the Optical Properties of DPH. Molecules, 25(18), 4264. https://doi.org/10.3390/molecules25184264