Valorization of Cork Using Subcritical Water
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Characterization of Granulated Cork
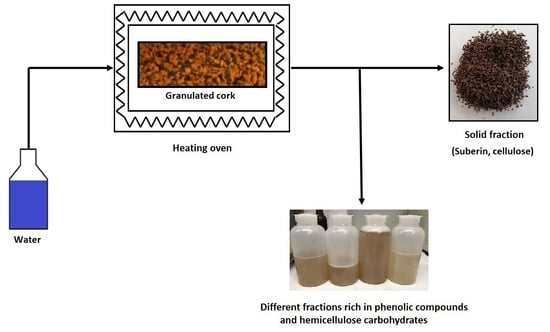
2.2. Efficiency of SBW Extraction/Hydrolysis
2.3. Antioxidant Activity
2.4. Suberin and Lignin Extraction
3. Materials and Methods
3.1. Materials
3.2. Chemical Characterization of Granulated Cork
3.3. Semi-Continuous SBW Extraction
3.4. Phenolic Analysis
3.5. Carbohydrate Analysis
3.6. Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pereira, H. Cork products and uses. In Cork: Biology Production and Uses; Pereira, H., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 11, pp. 243–261. [Google Scholar] [CrossRef]
- Cork Year Book 19/20. Available online: https://www.apcor.pt/portfolio-posts/boletim-estatistico-1920 (accessed on 7 July 2020).
- Mestre, A.; Gil, L. Cork for sustainable product design. Cienc. Tecnol. Mater. 2011, 23, 52–63. [Google Scholar]
- Matos, A.M.; Nunes, S.; Coutinho, J.S. Cork waste in cement based materials. Mater. Des. 2015, 85, 230–239. [Google Scholar] [CrossRef]
- Carriço, C.; Ribeiro, H.M.; Marto, J. Converting cork by-products to eco-friendly cork bioactive ingredients: Novel pharmaceutical and cosmetics applications. Ind. Crops Prod. 2018, 125, 72–84. [Google Scholar] [CrossRef]
- Silva, S.P.; Sabino, M.A.; Fernandes, E.M.; Correlo, V.M.; Boesel, L.F.; Reis, R.L. Cork: Properties, capabilities and applications. Int. Mater. Rev. 2005, 50, 345–365. [Google Scholar] [CrossRef] [Green Version]
- Reis, S.F.; Lopes, P.; Roseira, I.; Cabral, M.; Mateus, N.; Freitas, V. Recovery of added value compounds from cork industry by-products. Ind. Crops Prod. 2019, 140, 111599. [Google Scholar] [CrossRef]
- Conde, E.; Cadahía, E.; García-Vallejo, M.C.; de Simón, B.F.; Adrados, J.R.G. Low Molecular Weight Polyphenols in Cork of Quercus suber. J. Agric. Food Chem. 1997, 45, 2695–2700. [Google Scholar] [CrossRef]
- Sen, A.; Miranda, I.; Santos, S.; Graça, J.; Pereira, H. The chemical composition of cork and phloem in the rhytidome of Quercus cerris bark. Ind. Crops Prod. 2010, 31, 417–422. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Pinto, P.C.R.O.; Silvestre, A.J.D.; Neto, C.P. Chemical composition and antioxidant activity of phenolic extracts of cork from Quercus suber L. Ind. Crops Prod. 2010, 31, 521–526. [Google Scholar] [CrossRef]
- Bouras, M.; Chadni, M.; Barba, F.J.; Grimi, N.; Bals, O.; Vorobiev, E. Optimization of microwave-assisted extraction of polyphenols from Quercus bark. Ind. Crops Prod. 2015, 77, 590–601. [Google Scholar] [CrossRef]
- Batista, M.; Rosete, M.; Ferreira, I.; Ferreira, J.; Duarte, C.; Matias, A.; Poejo, J.; Crespo, J.; Valério, R. Cork Hydroglycolic Extract, Method for Preparing Same, Formulations Comprising Said Extract and Use Thereof. Patent WO2015/152746, 8 October 2015. [Google Scholar]
- Galbe, M.; Wallberg, O. Pretreatment for biorefineries: A review of common methods for efficient utilisation of lignocellulosic materials. Biotechnol. Biofuels 2019, 12, 294. [Google Scholar] [CrossRef] [Green Version]
- Brunner, G. Near Critical and Supercritical Water, Part I: Hydrolytic and Hydrothermal Processes. J. Supercrit. Fluids 2009, 47, 373–381. [Google Scholar] [CrossRef]
- Pereira, H. Chemical composition and variability of cork. Wood Sci. Technol. 1988, 22, 211–218. [Google Scholar] [CrossRef]
- Jové, P.; Olivella, M.A.; Cano, L. Study of the variability in chemical composition of bark layers of Quercus suber L. from different production areas. BioResources 2011, 6, 1806–1815. [Google Scholar]
- Conde, E.; Cadahia, E.; Garcia-Vallejo, M.C.; Gonzhlez-Adrados, J.R. Chemical characterization of reproduction cork from Spanish Quercus suber. J. Wood Chem. Technol. 1998, 18, 447–469. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Villaverde, J.J.; Sousa, A.F.; Neto, C.P.; Silvestre, A.J.D.; Coelho, J.J.F. Phenolic composition and antioxidant activity of industrial cork by-products. Ind. Crops Prod. 2013, 47, 262–269. [Google Scholar] [CrossRef]
- Pedras, B.; Salema-Oom, M.; Sá-Nogueira, I.; Simões, P.; Paiva, A.; Barreiros, S. Valorization of white wine grape pomace through application of subcritical water: Analysis of extraction, hydrolysis and biological activity of the extracts obtained. J. Supercrit. Fluids 2017, 128, 138–144. [Google Scholar] [CrossRef]
- Templeton, D.W.; Quinn, M.; Wychen, S.V.; Hyman, D.; Laurens, L.M.L. Separation and quantification of microalgal carbohydrates. J. Chromatogr. A 2012, 1270, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Cetinkol, O.P.; Smith-Moritz, A.; Cheng, G.; Lao, L.; George, A.; Hong, K.; Henry, R.; Simons, B.A.; Heazlewood, J.L.; Holmes, B.M. Structural and Chemical Characterization of Hardwood from Tree Species with Applications as Bioenergy Feedstocks. PLoS ONE 2012, 7, e52820. [Google Scholar] [CrossRef] [Green Version]
- Bandura, A.V.; Lvov, S.N. The Ionization Constant of Water over Wide Ranges of Temperature and Density. J. Phys. Chem. Ref. Data 2006, 35, 15–30. [Google Scholar] [CrossRef] [Green Version]
- Möller, M.; Nilges, P.; Harnisch, F.; Schröder, U. Subcritical Water as Reaction Environment: Fundamentals of Hydrothermal Biomass Transformation. ChemSusChem 2011, 4, 566–579. [Google Scholar] [CrossRef]
- Uematsu, M.; Franck, E.U. Static Dielectric Constant of Water and Steam. J. Phys. Chem. Ref. Data 1980, 9, 1291–1306. [Google Scholar] [CrossRef]
- Alfei, S.; Turrini, F.; Catena, S.; Zunin, P.; Parodi, B.; Zuccari, G.; Pittaluga, A.M.; Boggia, R. Preparation of ellagic acid micro and nano formulations with amazingly increased water solubility by its entrapment in pectin or non-PAMAM dendrimers suitable for clinical applications. New J. Chem. 2019, 43, 2438–2448. [Google Scholar] [CrossRef]
- Mislata, A.M.; Puxeu, M.; Ferrer-Gallego, R. Aromatic Potential and Bioactivity of Cork Stoppers and Cork By-Products. Foods 2020, 9, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedras, B.; Nascimento, M.; Sá-Nogueira, I.; Simões, P.; Paiva, A.; Barreiros, S. Semi-continuous extraction/hydrolysis of spent coffee grounds with subcritical water. J. Ind. Eng. Chem. 2019, 72, 453–456. [Google Scholar] [CrossRef]
- Aroso, I.M.; Araujo, A.R.; Fernandes, J.P.; Santos, T.; Batista, M.T.; Pires, R.A.; Mano, J.F.; Reis, R.L. Hydroalcoholic extracts from the bark of Quercus suber L. (Cork): Optimization of extraction conditions, chemical composition and antioxidant potential. Wood Sci. Technol. 2017, 51, 855–872. [Google Scholar] [CrossRef]
- Cordeiro, N.; Belgacem, M.N.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A. Cork suberin as a new source of chemicals. 1. Isolation and chemical characterization of its composition. Int. J. Biol. Macromol. 1998, 22, 71–80. [Google Scholar] [CrossRef]
- Laboratory Analytical Procedure (LAP); Hames, B.; Scarlata, C.; Sluiter, A. Determination of Protein Content in Biomass; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2008. [Google Scholar]
- Järvinen, R.; Silvestre, A.J.D.; Holopainen, H.; Kaimainen, M.; Nyyssölä, A.; Gil, A.M.; Neto, C.P.; Lehtinen, P.; Buchert, J.; Kallio, H. Suberin of Potato (Solanum tubersoum Var. Nikola): Comparison of the Effect of Cutinase CcCut1 with Chemical Depolymerization. J. Agric. Food. Chem. 2009, 57, 9016–9027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laboratory Analytical Procedure (LAP); Sluiter, A.; Hames, B.; Ruiz, R.; Scarlate., C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2012. [Google Scholar]
- Yilmaz, F.M.; Karaaslan, M.; Vardin, H. Optimization of extraction parameters on the isolation of phenolic compounds from sour cherry (Prunus cerasus L.) pomace. J. Food Sci. Technol. 2015, 52, 2851–2859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivaraman, T.; Kumar, T.K.S.; Jayaraman, G.; Yu, C. The Mechanism of 2,2,2-Trichloroacetic Acid-Induced Protein Precipitation. J. Protein Chem. 1997, 16, 291–297. [Google Scholar] [CrossRef]
- Fernandes, A.; Sousa, A.; Mateus, N.; Cabral, M.; de Freitas, V. Analysis of phenolic compounds in cork from Quercus suber L. by HPLC–DAD/ESI–MS. Food Chem. 2011, 125, 1398–1405. [Google Scholar] [CrossRef]
- Dionex Corporation. Product Manual for Dionex CarboPac MA1, PA1, PA10 and PA100 Columns. Available online: http://www.Dionex.com/liu.umd.edu/files/4375-man-031824-08-CarboPac-Combined-May10.pdf (accessed on 10 June 2020).
- Brás, T.; Guerreiro, O.; Duarte, M.F.; Neves, L.A. Impact of extraction parameters and concentration by nanofiltration on the recovery of phenolic compounds from Cynara cardunculus var. altilis: Assessment of antioxidant activity. Ind. Crops Prod. 2015, 67, 137–142. [Google Scholar] [CrossRef] [Green Version]



| Component | wt.% |
|---|---|
| Ashes | 0.89 ± 0.02 |
| Extractives | 11.4 ± 2.2 |
| n-Hexane | 3.3 ± 0.4 |
| Ethanol | 4.4 ± 0.8 |
| Water | 3.7 ± 1.0 |
| Suberin | 41.0 ± 3.4 |
| Lignin | 24.9 ± 3.4 |
| Soluble | 1.6 ± 0.2 |
| Insoluble | 23.3 ± 3.2 |
| Carbohydrates | 18.4 ± 5.2 |
| Cellulose 1 | 7.8 ± 2.1 |
| Hemicellulose | 10.6 ± 3.1 |
| Protein | 3.2 ± 0.1 |
| Temperature (°C) | Extraction Yield (g/100 g Cork) | Yield of Carbohydrates (g/100 g Cork) | Yield of Phenolics (g/100 g Cork) |
|---|---|---|---|
| 120 | 2.17 ± 0.07 | 0.59 ± 0.02 | 0.73 ± 0.04 |
| 150 | 10.5 ± 0.3 | 2.86 ± 0.16 | 2.15 ± 0.10 |
| 200 | 17.0 ± 0.5 | 7.27 ± 0.06 | 3.76 ± 0.18 |
| Target Temperature (°C) | Temperature of Sample Collection (°C) | Extraction Yield (g/100 g Cork) | Yield of Carbohydrates (g/100 g Cork) | Yield of Phenolics (g/100 g Cork) |
|---|---|---|---|---|
| 120 °C | <50 | 0.3 | 0.07 | 0.07 |
| 50–120 | 1.6 | 0.44 | 0.54 | |
| 120 | 2.2 | 0.59 | 0.73 | |
| 150 °C | <50 | 0.7 | 0.09 | 0.17 |
| 50–150 | 7.3 | 1.43 | 1.61 | |
| 150 | 10.5 | 2.86 | 2.15 | |
| 200 °C | <50 | 0.3 | 0.09 | 0.08 |
| 50–120 | 1.2 | 0.25 | 0.31 | |
| 120–200 | 12.8 | 6.49 | 2.81 | |
| 200 | 17.0 | 7.27 | 3.76 |
| T (°C) | Phenolic Content (mg/gextract) | Phenolic Content (μg/gdry cork) | ||||||
|---|---|---|---|---|---|---|---|---|
| Ellagic Acid | Gallic Acid | Caffeic Acid | Ferulic Acid | Ellagic Acid | Gallic Acid | Caffeic Acid | Ferulic Acid | |
| <50 | 4.7 ± 0.8 | 0.6 ± 0.1 | 0.8 ± 0.1 | 14.9 ± 2.6 | 2.0 ± 0.4 | 2.6 ± 0.5 | ||
| 50–120 | 4.9 ± 0.9 | 0.5 ± 0.1 | 0.6 ± 0.1 | 61.2 ± 10.9 | 6.5 ± 1.1 | 7.5 ± 1.4 | ||
| 120–200 | 1.4 ± 0.2 | 4.5 ± 0.8 | 147.2 ± 26.2 | 524.6 ± 93.4 | ||||
| 200 | 2.1 ± 0.4 | 3.0 ± 0.5 | 0.13 ± 0.02 | 0.4 ± 0.1 | 86.8 ± 15.5 | 123.9 ± 22.1 | 5.5 ± 0.9 | 14.8 ± 2.7 |
| Temperature (°C) | EC50 (mg extract/mg DPPH) | TPC (g/100 g extract) |
|---|---|---|
| <50 | 0.406 ± 0.007 | 26.7 ± 2.1 |
| 50–120 | 0.253 ± 0.001 | 25.6 ± 0.2 |
| 120–200 | 0.459 ± 0.006 | 21.6 ± 1.3 |
| 200 | 0.510 ± 0.004 | 22.6 ± 0.4 |
Sample Availability: Samples of the extracts are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cunha, M.; Lourenço, A.; Barreiros, S.; Paiva, A.; Simões, P. Valorization of Cork Using Subcritical Water. Molecules 2020, 25, 4695. https://doi.org/10.3390/molecules25204695
Cunha M, Lourenço A, Barreiros S, Paiva A, Simões P. Valorization of Cork Using Subcritical Water. Molecules. 2020; 25(20):4695. https://doi.org/10.3390/molecules25204695
Chicago/Turabian StyleCunha, Mónica, Ana Lourenço, Susana Barreiros, Alexandre Paiva, and Pedro Simões. 2020. "Valorization of Cork Using Subcritical Water" Molecules 25, no. 20: 4695. https://doi.org/10.3390/molecules25204695
APA StyleCunha, M., Lourenço, A., Barreiros, S., Paiva, A., & Simões, P. (2020). Valorization of Cork Using Subcritical Water. Molecules, 25(20), 4695. https://doi.org/10.3390/molecules25204695