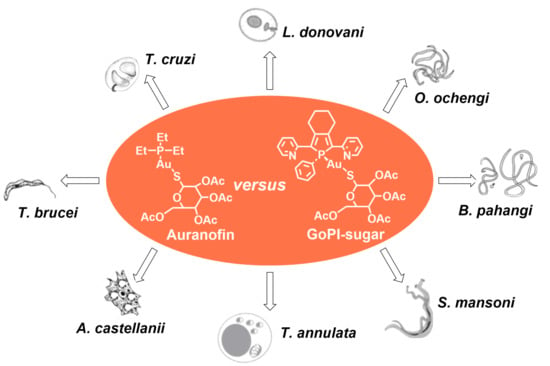
Repurposing Auranofin and Evaluation of a New Gold(I) Compound for the Search of Treatment of Human and Cattle Parasitic Diseases: From Protozoa to Helminth Infections
Abstract
:1. Introduction
2. Results
2.1. Synthesis of GoPI-Sugar
2.2. Inhibitory Activities of Gold(I) Complexes against Members of the NADPH-Dependent Disulfide Reductase Family
2.3. Anthelmintic Activity of Gold(I) Complexes
2.3.1. Schistosoma mansoni
2.3.2. Filarial Parasites
2.4. Anti-Kinetoplastid Activity of Gold(I) Complexes
2.4.1. Trypanothione Reductase from Kinetoplastidae
2.4.2. Trypanosoma b. gambiense and T. b. brucei
2.4.3. Trypanosoma cruzi and Leishmania infantum
2.4.4. Leishmania donovani
2.5. Anti-Amoeba Activity of Gold(I) Complexes
Acanthamoeba castellanii
2.6. Activity of GoPI-Sugar against Theileria-Transformed Leukocytes
2.7. Cytotoxicity Activity of Gold(I) Complexes
3. Discussion
4. Materials and Methods
4.1. Reagents and Tested Gold(I) Complexes
4.2. Helminth TGR Enzyme Assays
4.3. In Vitro Activity on Schistosoma and Filarial Worms
4.4. In Vitro Drug Activity against T. b. brucei, T. cruzi and L. infantum
4.5. In Vitro Drug Activity against L. donovani and A. castellanii
4.6. In Vitro Drug Activity against Theileria-Transformed Leukocyte
4.7. Cytotoxicity on Mammalian Cells
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Higby, G.J. Gold in medicine: A review of its use in the West before 1900. Gold Bull. 1982, 15, 130–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, R. Über bakteriologische Forschung. Dtsch. Med. Wochenstr. 1890, 16, 756–757. [Google Scholar]
- Forestier, J. Rheumatoid arthritis and its treatment by gold salts. Lancet 1934, 224, 646–648. [Google Scholar] [CrossRef]
- Forestier, J. Rheumatoid arthritis and its treatment by gold salts: The results of six years’ experience. J. Lab. Clin. Med. 1935, 20, 827–840. [Google Scholar]
- Kean, W.F.; Hart, L.; Buchanan, W.W. Auranofin. Br. J. Rheumatol. 1997, 36, 560–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messori, L.; Marcon, G. Gold complexes in the treatment of rheumatoid arthritis. Met. Ions Biol. Syst. 2004, 41, 279–304. [Google Scholar] [PubMed]
- Mirabelli, C.K.; Johnson, R.K.; Hill, D.T.; Faucette, L.F.; Girard, G.R.; Kuo, G.Y.; Sung, C.M.; Crooke, S.T. Correlation of the in vitro cytotoxic and in vivo antitumor activities of gold(I) coordination complexes. J. Med. Chem. 1986, 29, 218–223. [Google Scholar] [CrossRef]
- Madeira, J.M.; Gibson, D.L.; Kean, W.F.; Klegeris, A. The biological activity of auranofin: Implications for novel treatment of diseases. Inflammopharmacology 2012, 20, 297–306. [Google Scholar] [CrossRef]
- Roder, C.; Thomson, M.J. Auranofin: Repurposing an old drug for a golden new age. Drugs R D 2015, 15, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Massai, L.; Messori, L.; Micale, N.; Schirmeister, T.; Maes, L.; Fregona, D.; Cinellu, M.A.; Gabbiani, C. Gold compounds as cysteine protease inhibitors: Perspectives for pharmaceutical application as antiparasitic agents. BioMetals 2017, 30, 313–320. [Google Scholar] [CrossRef]
- Gromer, S.; Arscott, L.D.; Williams, C.H., Jr.; Schirmer, R.H.; Becker, K. Human placenta thioredoxin reductase. Isolation of the selenoenzyme, steady state kinetics, and inhibition by therapeutic gold compounds. J. Biol. Chem. 1998, 273, 20096–20101. [Google Scholar] [CrossRef] [Green Version]
- Deponte, M.; Urig, S.; Arscott, L.D.; Fritz-Wolf, K.; Réau, R.; Herold-Mende, C.; Koncarevic, S.; Meyer, M.; Davioud-Charvet, E.; Ballou, D.P.; et al. Mechanistic studies on a novel, highly potent gold-phosphole inhibitor of human glutathione reductase. J. Biol. Chem. 2005, 280, 20628–20637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urig, S.; Fritz-Wolf, K.; Reau, R.; Herold-Mende, C.; Toth, K.; Davioud-Charvet, E.; Becker, K. Undressing of phosphine gold(I) complexes as irreversible inhibitors of human disulfide reductases. Angew. Chem. Int. Ed. Engl. 2006, 45, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Viry, E.; Battaglia, E.; Deborde, V.; Müller, T.; Réau, R.; Davioud-Charvet, E.; Bagrel, D. A sugar-modified phosphole gold complex with antiproliferative properties acting as a thioredoxin reductase inhibitor in MCF-7 cells. ChemMedChem 2008, 3, 1667–1670. [Google Scholar] [CrossRef] [PubMed]
- Jortzik, E.; Farhadi, M.; Ahmadi, R.; Tóth, K.; Lohr, J.; Helmke, B.M.; Kehr, S.; Unterberg, A.; Ott, I.; Gust, R.; et al. Antiglioma activity of GoPI-sugar, a novel gold(I)-phosphole inhibitor: Chemical synthesis, mechanistic studies, and effectiveness in vivo. Biochim. Biophys. Acta 2014, 1844, 1415–1426. [Google Scholar] [CrossRef]
- Fan, C.; Zheng, W.; Fu, X.; Li, X.; Wong, Y.S.; Chen, T. Enhancement of auranofin-induced lung cancer cell apoptosis by selenocystine, a natural inhibitor of TrxR1 in vitro and in vivo. Cell Death Dis. 2014, 5, 1191. [Google Scholar] [CrossRef] [Green Version]
- Fiskus, W.; Saba, N.; Shen, M.; Ghias, M.; Liu, J.; Gupta, S.D.; Chauhan, L.; Rao, R.; Gunewardena, S.; Schorno, K.; et al. Auranofin induces lethal oxidative and endoplasmic reticulum stress and exerts potent preclinical activity against chronic lymphocytic leukemia. Cancer Res. 2014, 74, 2520–2532. [Google Scholar] [CrossRef] [Green Version]
- Marzano, C.; Gandin, V.; Folda, A.; Scutari, G.; Bindoli, A.; Rigobello, M.P. Inhibition of thioredoxin reductase by auranofin induces apoptosis in cisplatin-resistant human ovarian cancer cells. Free Radic. Biol. Med. 2007, 42, 872–881. [Google Scholar] [CrossRef]
- Crooke, S.T.; Mirabelli, C.K. Molecular mechanisms of action of auranofin and other gold complexes as related to their biologic activities. Am. J. Med. 1983, 75, 109–113. [Google Scholar] [CrossRef]
- Dessolin, J.; Biot, C.; Davioud-Charvet, E. Bromination studies of the 2,3-dimethylnaphthazarin core allowing easy access to naphthazarin derivatives. J. Org. Chem. 2001, 66, 5616–5619. [Google Scholar] [CrossRef]
- Irmler, A.; Bechthold, A.; Davioud-Charvet, E.; Hofmann, V.; Réau, R.; Gromer, S.; Schirmer, R.H.; Becker, K. Disulfide reductases–current developments. In Flavins and Flavoproteins 2002; Chapman, S.K., Perham, R.N., Scrutton, N.S., Eds.; Agency for Scientific Publications: Berlin, Germany, 2002; pp. 803–815. [Google Scholar]
- Millet, R.; Urig, S.; Jacob, J.; Amtmann, E.; Moulinoux, J.P.; Gromer, S.; Becker, K.; Davioud-Charvet, E. Synthesis of 5-nitro-2-furancarbohydrazides and their cis-diamminedichloroplatinum complexes as bitopic and irreversible human thioredoxin reductase inhibitors. J. Med. Chem. 2005, 48, 7024–7039. [Google Scholar] [CrossRef] [PubMed]
- Davioud-Charvet, E.; McLeish, M.J.; Veine, D.M.; Giegel, D.; Arscott, L.D.; Andricopulo, A.D.; Becker, K.; Müller, S.; Schirmer, R.H.; Williams, C.H., Jr.; et al. Mechanism-based inactivation of thioredoxin reductase from Plasmodium falciparum by Mannich bases. Implication for cytotoxicity. Biochemistry 2003, 42, 13319–13330. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, A.N.; Davioud-Charvet, E.; Sayed, A.A.; Califf, L.L.; Dessolin, J.; Arnér, E.S.; Williams, D.L. Thioredoxin glutathione reductase from Schistosoma mansoni: An essential parasite enzyme and a key drug target. PLoS Med. 2007, 4, e206, Erratum in: PLoS Med. 2007, 4, e264. [Google Scholar]
- Andricopulo, A.D.; Akoachere, M.B.; Krogh, R.; Nickel, C.; McLeish, M.J.; Kenyon, G.L.; Arscott, L.D.; Williams, C.H., Jr.; Davioud-Charvet, E.; Becker, K. Specific inhibitors of Plasmodium falciparum thioredoxin reductase as potential antimalarial agents. Bioorg. Med. Chem. Lett. 2006, 16, 2283–2922. [Google Scholar] [CrossRef]
- Abrams, M.J.; Murrer, B.A. Metal compounds in therapy and diagnosis. Science 1993, 261, 725–730. [Google Scholar] [CrossRef]
- Angelucci, F.; Sayed, A.A.; Williams, D.L.; Boumis, G.; Brunori, M.; Dimastrogiovanni, D.; Miele, A.E.; Pauly, F.; Bellelli, A. Inhibition of Schistosoma mansoni thioredoxin-glutathione reductase by auranofin: Structural and kinetic aspects. J. Biol. Chem. 2009, 284, 28977–28985. [Google Scholar] [CrossRef] [Green Version]
- Roberts, J.R.; Xiao, J.; Schliesman, B.; Parsons, D.J.; Shaw, C.F., 3rd. Kinetics and Mechanism of the Reaction between Serum Albumin and Auranofin (and Its Isopropyl Analogue) In Vitro. Inorg. Chem. 1996, 35, 424–433. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.; Taylor, P.; Dornan, J.; Robinson, S.P.; Walkinshaw, M.D.; Sadler, P.J. First crystal structure of a medicinally relevant gold protein complex: Unexpected binding of [Au(PEt3)](+) to histidine. Angew. Chem. Int. Ed. 2000, 39, 2931–2934. [Google Scholar] [CrossRef]
- Da Silva, M.T.; Silva-Jardim, I.; Portapilla, G.B.; de Lima, G.M.; Costa, F.C.; Anibal, F.F.; Thiemann, O.H. In vivo and in vitro auranofin activity against Trypanosoma cruzi: Possible new uses for an old drug. Exp. Parasitol. 2016, 166, 189–193. [Google Scholar] [CrossRef]
- Ilari, A.; Baiocco, P.; Messori, L.; Fiorillo, A.; Boffi, A.; Gramiccia, M.; Di Muccio, T.; Colotti, G. A gold-containing drug against parasitic polyamine metabolism: The X-ray structure of trypanothione reductase from Leishmania infantum in complex with auranofin reveals a dual mechanism of enzyme inhibition. Amino Acids 2012, 42, 803–811. [Google Scholar] [CrossRef] [Green Version]
- Sannella, A.R.; Casini, A.; Gabbiani, C.; Messori, L.; Bilia, A.R.; Vincieri, F.F.; Majori, G.; Severini, C. New uses for old drugs. Auranofin, a clinically established antiarthritic metallodrug, exhibits potent antimalarial effects in vitro: Mechanistic and pharmacological implications. FEBS Lett. 2008, 582, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Tejman-Yarden, N.; Miyamoto, Y.; Leitsch, D.; Santini, J.; Debnath, A.; Gut, J.; McKerrow, J.H.; Reed, S.L.; Eckmann, L. A reprofiled drug, auranofin, is effective against metronidazole-resistant Giardia lamblia. Antimicrob. Agents Chemother. 2013, 57, 2029–2035. [Google Scholar] [CrossRef] [Green Version]
- Bonilla, M.; Denicola, A.; Novoselov, S.V.; Turanov, A.A.; Protasio, A.; Izmendi, D.; Gladyshev, V.N.; Salinas, G. Platyhelminth mitochondrial and cytosolic redox homeostasis is controlled by a single thioredoxin glutathione reductase and dependent on selenium and glutathione. J. Biol. Chem. 2008, 283, 17898–17907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debnath, A.; Parsonage, D.; Andrade, R.M.; He, C.; Cobo, E.R.; Hirata, K.; Chen, S.; García-Rivera, G.; Orozco, E.; Martínez, M.B.; et al. A high-throughput drug screen for Entamoeba histolytica identifies a new lead and target. Nat. Med. 2012, 18, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Bulman, C.A.; Bidlow, C.M.; Lustigman, S.; Cho-Ngwa, F.; Williams, D.; Rascón, A.A., Jr.; Tricoche, N.; Samje, M.; Bell, A.; Suzuki, B.; et al. Repurposing auranofin as a lead candidate for treatment of lymphatic filariasis and onchocerciasis. PLoS Negl. Trop. Dis. 2015, 9, e0003534. [Google Scholar] [CrossRef] [PubMed]
- Sonogashira, K. Development of Pd–Cu catalyzed cross-coupling of terminal acetylenes with sp2-carbon halides. J. Organomet. Chem. 2002, 53, 46–49. [Google Scholar] [CrossRef]
- Aranda Perez, A.I.; Biet, T.; Graule, S.; Agou, T.; Lescop, C.; Branda, N.R.; Crassous, J.; Réau, R. Chiral and extended π-conjugated bis(2-pyridyl)phospholes as assembling N,P,N pincers for coordination-driven synthesis of supramolecular [2,2]paracyclophane analogues. Chemistry 2011, 17, 1337–1351. [Google Scholar] [CrossRef]
- Hay, C.; Hissler, M.; Fischmeister, C.; Rault-Berthelot, J.; Toupet, L.; Nyulászi, L.; Réau, R. Phosphole- containing pi-conjugated systems: From model molecules to polymer films on electrodes. Chemistry 2001, 7, 4222–4236. [Google Scholar] [CrossRef]
- Yan, X.; Xi, C. Conversion of zirconacyclopentadienes into metalloles: Fagan-Nugent reaction and beyond, Acc. Chem. Res. 2015, 48, 935–946. [Google Scholar] [CrossRef]
- King, C.H. Parasites and poverty: The case of schistosomiasis. Acta Trop. 2010, 113, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Bergquist, R.; Elmorshedy, H. Artemether and Praziquantel: Origin, Mode of Action, Impact, and Suggested Application for Effective Control of Human Schistosomiasis. Trop. Med. Infect. Dis. 2018, 3, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alger, H.M.; Williams, D.L. The disulfide redox system of Schistosoma mansoni and the importance of a multifunctional enzyme, thioredoxin glutathione reductase. Mol. Biochem. Parasitol. 2002, 121, 129–139. [Google Scholar] [CrossRef]
- Williams, D.L.; Bonilla, M.; Gladyshev, V.N.; Salinas, G. Thioredoxin glutathione reductase-dependent redox networks in platyhelminth parasites. Antioxid. Redox Signal. 2013, 19, 735–745. [Google Scholar] [CrossRef]
- Bonilla, M.; Denicola, A.; Marino, S.M.; Gladyshev, V.N.; Salinas, G. Linked thioredoxin-glutathione systems in platyhelminth parasites: Alternative pathways for glutathione reduction and deglutathionylation. J. Biol. Chem. 2011, 286, 4959–4967. [Google Scholar] [CrossRef] [Green Version]
- Prast-Nielsen, S.; Huang, H.-H.; Williams, D.L. Thioredoxin glutathione reductase: Its role in redox biology and potential as a target for drugs against neglected diseases. Biochim. Biophys. Acta 2011, 1810, 1262–1271. [Google Scholar] [CrossRef] [Green Version]
- Angelucci, F.; Dimastrogiovanni, D.; Boumis, G.; Brunori, M.; Miele, A.E.; Saccoccia, F.; Bellelli, A. Mapping the catalytic cycle of Schistosoma mansoni thioredoxin glutathione reductase by X-ray crystallography. J. Biol. Chem. 2010, 285, 32557–32567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.-H.; Day, L.; Cass, C.L.; Ballou, D.P.; Williams, C.H., Jr.; Williams, D.L. Investigations of the catalytic mechanism of thioredoxin glutathione reductase from Schistosoma mansoni. Biochemistry 2011, 50, 5870–5882. [Google Scholar] [CrossRef] [Green Version]
- Salinas, G.; Gao, W.; Wang, Y.; Bonilla, M.; Yu, L.; Novikov, A.; Virginio, V.G.; Ferreira, H.B.; Vieites, M.; Gladyshev, V.N.; et al. The Enzymatic and Structural Basis for Inhibition of Echinococcus granulosus Thioredoxin Glutathione Reductase by Gold(I). Antioxid. Redox Signal. 2017, 27, 1491–1504. [Google Scholar] [CrossRef]
- Martínez-González, J.J.; Guevara-Flores, A.; Rendón, J.L.; Arenal, I.P.D. Auranofin-induced oxidative stress causes redistribution of the glutathione pool in Taenia crassiceps cysticerci. Mol. Biochem. Parasitol. 2015, 27, 16–25. [Google Scholar] [CrossRef]
- Martínez-González, J.J.; Guevara-Flores, A.; Alvarez, G.; Rendón-Gómez, J.L.; Del Arenal, I.P. In vitro killing action of auranofin on Taenia crassiceps metacestode (cysticerci) and inactivation of thioredoxin-glutathione reductase (TGR). Parasitol Res. 2010, 107, 227–231. [Google Scholar] [CrossRef]
- Rigobello, M.P.; Messori, L.; Marcon, G.; Agostina Cinellu, M.; Bragadin, M.; Folda, A.; Scutari, G.; Bindoli, A. Gold complexes inhibit mitochondrial thioredoxin reductase: Consequences on mitochondrial functions. J. Inorg. Biochem. 2004, 98, 1634–1641. [Google Scholar] [CrossRef]
- Capparelli, E.V.; Bricker-Ford, R.; Rogers, M.J.; McKerrow, J.H.; Reed, S.L. Phase I Clinical Trial Results of Auranofin, a Novel Antiparasitic Agent. Antimicrob. Agents Chemother. 2016, 61, e01947–e02016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lombardo, F.C.; Pasche, V.; Panic, G.; Endriss, Y.; Keiser, J. Life cycle maintenance and drug-sensitivity assays for early drug discovery in Schistosoma mansoni. Nat. Protoc. 2019, 14, 461–481. [Google Scholar] [CrossRef]
- Marcellino, C.; Gut, J.; Lim, K.C.; Singh, R.; McKerrow, J.; Sakanari, J. WormAssay: A novel computer application for whole plate screening of macroscopic parasites. PLoS Negl. Trop. Dis. 2012, 6, e1494. [Google Scholar] [CrossRef]
- Gardon, J.; Gardon-Wendel, N.; Demanga, N.; Kamgno, J.; Chippaux, J.P.; Boussinesq, M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet 1997, 350, 18–22. [Google Scholar] [CrossRef]
- Boussinesq, M.; Gardon, J.; Gardon-Wendel, N.; Chippaux, J.P. Clinical picture, epidemiology and outcome of Loa-associated serious adverse events related to mass ivermectin treatment of onchocerciasis in Cameroon. Filaria J. 2003, 2 (Suppl 1), S4. [Google Scholar] [CrossRef] [Green Version]
- Fairlamb, A.H.; Cerami, A. Metabolism and functions of trypanothione in the Kinetoplastida. Annu. Rev. Microbiol. 1992, 46, 695–729. [Google Scholar] [CrossRef]
- Krieger, S.; Schwarz, W.; Ariyanayagam, M.R.; Fairlamb, A.H.; Krauth-Siegel, R.L.; Clayton, C. Trypanosomes lacking trypanothione reductase are avirulent and show increased sensitivity to oxidative stress. Mol. Microbiol. 2000, 35, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Gendron, T.; Lanfranchi, D.A.; Davioud-Charvet, E. Redox-active agents in reactions involving the trypanothione/trypanothione reductase-based system to fight kinetoplastidal parasites. In Drug Discovery in Infectious Diseases; Selzer, P.M., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; Volume 4, pp. 405–428. [Google Scholar]
- Lee, B.; Bauer, H.; Melchers, J.; Ruppert, T.; Rattray, L.; Yardley, V.; Davioud-Charvet, E.; Krauth-Siegel, R.L. Irreversible inactivation of trypanothione reductase by unsaturated Mannich bases: A divinyl ketone as key intermediate. J. Med. Chem. 2005, 48, 7400–7410. [Google Scholar] [CrossRef]
- Lazarin-Bidóia, D.; Desoti, V.C.; Martins, S.C.; Ribeiro, F.M.; Ud Din, Z.; Rodrigues-Filho, E.; Ueda-Nakamura, T.; Nakamura, C.V.; de Oliveira Silva, S. Dibenzylideneacetones Are Potent Trypanocidal Compounds That Affect the Trypanosoma cruzi Redox System. Antimicrob. Agents Chemother. 2015, 60, 890–903. [Google Scholar] [CrossRef] [Green Version]
- Cheikh-Ali, Z.; Caron, J.; Cojean, S.; Bories, C.; Couvreur, P.; Loiseau, P.M.; Desmaële, D.; Poupon, E.; Champy, P. “Squalenoylcurcumin” nanoassemblies as water-dispersible drug candidates with antileishmanial activity. Chem. Med. Chem. 2015, 10, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Fairlamb, A.H.; Henderson, G.B.; Cerami, A. Trypanothione is the primary target for arsenical drugs against African trypanosomes. Proc. Natl. Acad. Sci. USA 1989, 86, 2607–2611. [Google Scholar] [CrossRef] [Green Version]
- Wyllie, S.; Cunningham, M.L.; Fairlamb, A.H. Dual action of antimonial drugs on thiol redox metabolism in the human pathogen Leishmania donovani. J. Biol. Chem. 2004, 279, 39925–39932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharlow, E.R.; Leimgruber, S.; Murray, S.; Lira, A.; Sciotti, R.J.; Hickman, M.; Hudson, T.; Leed, S.; Caridha, T.; Barrios, A.M.; et al. Auranofin is an apoptosis-stimulating agent with in vitro and in vivo anti-leishmanial activity. ACS Chem. Biol. 2014, 9, 663–672. [Google Scholar] [CrossRef]
- Ondarza, R.N.; Iturbe, A.; Hernández, E.; Hurtado, G. Thiol compounds from a free-living pathogenic opportunistic amoeba, Acanthamoeba polyphaga. Biotechnol. Appl. Biochem. 2002, 36, 195–204. [Google Scholar] [CrossRef]
- Ondarza, R.N.; Iturbe, A.; Hernández, E. The effects by neuroleptics, antimycotics and antibiotics on disulfide reducing enzymes from the human pathogens Acanthamoeba polyphaga and Naegleria fowleri. Exp. Parasitol. 2007, 115, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Taravaud, A.; Loiseau, P.M.; Pomel, S. In vitro evaluation of antimicrobial agents on Acanthamoeba sp. and evidence of a natural resilience to amphotericin B. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 328–336. [Google Scholar] [CrossRef]
- Chaussepied, M.; Langsley, G. Theileria transformation of bovine leukocytes: A parasite model for the study of lymphoproliferation. Res. Immunol. 1996, 147, 127–138. [Google Scholar] [CrossRef]
- Dobbelaere, D.; Heussler, V. Transformation of leukocytes by Theileria parva and T. annulata. Annu. Rev. Microbiol. 1999, 53, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Darghouth, M.A. Review on the experience with live attenuated vaccines against tropical theileriosis in Tunisia: Considerations for the present and implications for the future. Vaccine 2008, 26, G4–G10. [Google Scholar] [CrossRef]
- Baylis, H.A.; Megson, A.; Hall, R. Infection with Theileria annulata induces expression of matrix metalloproteinase 9 and transcription factor AP-1 in bovine leucocytes. Mol. Biochem. Parasitol. 1995, 69, 211–222. [Google Scholar] [CrossRef]
- Hall, R.; Ilhan, T.; Kirvar, E.; Wilkie, G.; Preston, P.M.; Darghouth, M.; Somerville, R.; Adamson, R. Mechanism(s) of attenuation of Theileria annulata vaccine cell lines. Trop. Med. Int. Health 1999, 4, A78–A84. [Google Scholar] [CrossRef] [PubMed]
- Shiels, B.; Langsley, G.; Weir, W.; Pain, A.; McKellar, S.; Dobbelaere, D. Alteration of host cell phenotype by Theileria annulata and Theileria parva: Mining for manipulators in the parasite genomes. Int. J. Parasitol. 2006, 36, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Chaussepied, M.; Janski, N.; Baumgartner, M.; Lizundia, R.; Jensen, K.; Weir, W.; Shiels, B.R.; Weitzman, J.B.; Glass, E.J.; Werling, D.; et al. TGF-b2 induction regulates invasiveness of Theileria-transformed leukocytes and disease susceptibility. PLoS Pathog. 2010, 6, e1001197. [Google Scholar] [CrossRef] [Green Version]
- Haidar, M.; Whitworth, J.; Noé, G.; Liu, W.; Vidal, M.; Langsley, G. TGF-β2 induces Grb2 to recruit PI3-K to TGF-RII that activates JNK/AP-1-signaling and augments invasiveness of Theileria-transformed macrophages. Sci. Rep. 2015, 5, 15688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haidar, M.; Echebli, N.; Ding, Y.; Kamau, E.; Langsley, G. Transforming growth factor β2 promotes transcription of COX2 and EP4, leading to a prostaglandin E2-driven autostimulatory loop that enhances virulence of Theileria annulata-transformed macrophages. Infect. Immun. 2015, 83, 1869–1880. [Google Scholar] [CrossRef] [Green Version]
- Dessauge, F.; Lizundia, R.; Baumgartner, M.; Chaussepied, M.; Langsley, G. Taking the Myc is bad for Theileria. Trends Parasitol. 2005, 21, 377–385. [Google Scholar] [CrossRef]
- Dessauge, F.; Hilaly, S.; Baumgartner, M.; Blumen, B.; Werling, D.; Langsley, G. c-Myc activation by Theileria parasites promotes survival of infected B-lymphocytes. Oncogene 2005, 24, 1075–1083. [Google Scholar] [CrossRef] [Green Version]
- Heussler, V.T.; Rottenberg, S.; Schwab, R.; Küenzi, P.; Fernandez, P.C.; McKellar, S.; Shiels, B.; Chen, Z.J.; Orth, K.; Wallach, D.; et al. Hijacking of host cell IKK signalosomes by the transforming parasite Theileria. Science 2002, 298, 1033–1036. [Google Scholar] [CrossRef]
- Lizundia, R.; Chaussepied, M.; Naissant, B.; Masse, G.X.; Quevillon, E.; Michel, F.; Monier, S.; Weitzman, J.-B.; Langsley, G. The JNK/AP-1 pathway upregulates expression of the recycling endosome rab11a gene in B cells transformed by Theileria. Cell Microbiol. 2007, 9, 1936–1945. [Google Scholar] [CrossRef]
- Adamson, R.; Logan, M.; Kinnaird, J.; Langsley, G.; Hall, R. Loss of matrix metalloproteinase 9 activity in Theileria annulata-attenuated cells is at the transcriptional level and is associated with differentially expressed AP-1 species. Mol. Biochem. Parasitol. 2000, 106, 51–61. [Google Scholar] [CrossRef]
- Chaussepied, M.; Lallemand, D.; Moreau, M.F.; Adamson, R.; Hall, R.; Langsley, G. Upregulation of Jun and Fos family members and permanent JNK activity lead to constitutive AP-1 activation in Theileria-transformed leukocytes. Mol. Biochem. Parasitol. 1998, 94, 215–226. [Google Scholar] [CrossRef]
- Metheni, M.; Echebli, N.; Chaussepied, M.; Ransy, C.; Chéreau, C.; Jensen, K.; Glass, E.; Batteux, F.; Bouillaud, F.; Langsley, G. The level of H₂O₂ type oxidative stress regulates virulence of Theileria-transformed leukocytes. Cell Microbiol. 2014, 16, 269–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanza, J.S.; Pomel, S.; Loiseau, P.M.; Frézard, F. Recent advances in amphotericin B delivery strategies for the treatment of leishmaniases. Expert Opin. Drug Deliv. 2019, 16, 1063–1079. [Google Scholar] [CrossRef]
- Sundar, S.; Chakravarty, J.; Agarwal, D.; Rai, M.; Murray, H.W. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. N. Engl. J. Med. 2010, 362, 504–512. [Google Scholar] [CrossRef]
- Gao, N.; Xing, C.; Wang, H.; Feng, L.; Zeng, X.; Mei, L.; Peng, Z. pH-Responsive Dual Drug-Loaded Nanocarriers Based on Poly (2-Ethyl-2-Oxazoline) Modified Black Phosphorus Nanosheets for Cancer Chemo/Photothermal Therapy. Front Pharmacol. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Tucker, M.S.; Karunaratne, L.B.; Lewis, F.A.; Freitas, T.C.; Liang, Y.-S. Schistosomiasis. Curr. Protoc. Immunol. 2013, 103, 19.1.1–19.1.58. [Google Scholar] [CrossRef]
- Hirumi, H.; Hirumi, K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 1989, 75, 985–989. [Google Scholar] [CrossRef] [Green Version]
- Räz, B.; Iten, M.; Grether-Bühler, Y.; Kaminsky, R.; Brun, R. The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T.b. rhodesiense and T.b. gambiense) in vitro. Acta Trop. 1997, 68, 139–147. [Google Scholar] [CrossRef]
- Buckner, F.S.; Verlinde, C.L.; La Flamme, A.C.; Van Voorhis, W.C. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing β-galactosidase. Antimicrob. Agents Chemother. 1996, 40, 2592–2597. [Google Scholar] [CrossRef] [Green Version]
- Sager, H.; Davis, W.C.; Dobbelaere, D.A.; Jungi, T.W. Macrophage-parasite relationship in theileriosis. Reversible phenotypic and functional dedifferentiation of macrophages infected with Theileria annulata. J. Leukoc. Biol. 1997, 61, 459–468. [Google Scholar] [CrossRef]
- Mao, W.; Daligaux, P.; Lazar, N.; Ha-Duong, T.; Cavé, C.; van Tilbeurgh, H.; Loiseau, P.M.; Pomel, S. Biochemical analysis of leishmanial and human GDP-Mannose Pyrophosphorylases and selection of inhibitors as new leads. Sci. Rep. 2017, 7, 751. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Enzymes | Human TrxR [13,15] | Human TrxR [11,13,14,15,52] | Human TrxR [11] | Human GR [11,12,13,15] | S. mansoni TGR [24] | L. infantum TR [31] | S. mansoni TGR | B. pahangi TrxR | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Substrates | hTrxC72S | DTNB | E. coli TrxS2 | GSSG | DTNB | GSSG | HED + GSSG | TS2 | DTNB | DTNB |
| Gold(I) Complexes IC50 (nM) (enzyme concentration, substrate concentration) | ||||||||||
| Auranofin | 20 (2 nM, 3 mM)2 | Ki = 2.0 and 3.0 nM (1.7 nM, at 50 and 75 TrxS2, respectively) 2 | 40,000 (1.5 nM, 1 mM) 2 | 7 (20 nM, 3 mM) 3 | 9 (20 nM, 100 µM) 3 | 6 (20 nM, 8 mM) 3 | Ki = 155 ± 35 nM (40 nM, 50–400 µM) 2 | 1.1 (20 nM, 3 mM) 3 | 0.5 (20 nM, 3 mM) 3 | |
| GoPI | 6.9 (24 nM, 20 µM) 1 7 (30 nM, 20 µM) 1 | 0.8 (4.8 nM, 3 mM) 1 1 (3.7 nM, 3 mM) 1 | 1 (1.4 nM, 100 µM) 1 2 (1.4-2.8 nM, 100 µM) 1 | |||||||
| GoPI-sugar | 4.3 ± 1.6 (30 nM, 20 µM) 1 | 0.49 ± 0.04 (3.7 nM, 3 mM) 1 | 88.5 ± 28 (1.4-2.8 nM, 100 µM) 1 | 12.5 (40 nM, 3 mM) 1 | 5.5 (100 nM, 3 mM) 1 | |||||
| Aurothioglucose | 65 (2 nM, 3 mM) 2 | >100,000 (1.5 nM, 1 mM) 2 | 70 (20 nM, 3 mM) 3 | 3000 (20 nM, 100 µM) 3 | 400 (20 nM, 8 mM) 3 | |||||
| Aurothiomalate | 280 (2 nM, 3 mM) 2,4 | 90 (20 nM, 3 mM) 3 | 50 (20 nM, 100 µM) 3 | 50 (20 nM, 8 mM) 3 | ||||||
| Compound | Conc. (μM) | Dead (%) Day 1 | Dead (%) Day 2 | Dead (%) Day 3 | Dead (%) Day 4 | Dead (%) Day 5 |
|---|---|---|---|---|---|---|
| Auranofin | 10 | 100 | ||||
| GoPI-sugar | 10 | 100 | ||||
| Auranofin | 5 | 100 | ||||
| GoPI-sugar | 5 | 100 | ||||
| Auranofin | 2.5 | 50 | 100 | |||
| GoPI-sugar | 2.5 | 0 | 0 | 0 | 50 | 100 |
| Auranofin | 1 | 0 | 0 | 0 | 0 | 0 |
| GoPI-sugar | 1 | 0 | 0 | 0 | 0 | 0 |
| Compound | Conc. (μM) | Inhibition of Motility | |||
|---|---|---|---|---|---|
| (%) Day 1 | (%) Day 2 | (%) Day 3 | (%) Day 6 | ||
| Auranofin | 10 | 99 | 100 | 99 | 98 |
| GoPI-sugar | 10 | 91 | 98 | 100 | 99 |
| Auranofin | 3 | 98 | 100 | 99 | 98 |
| GoPI-sugar | 3 | 24 | 41 | 40 | 96 |
| Auranofin | 1 | 25 | 45 | 48 | 98 |
| GoPI-sugar | 1 | 20 | 16 | 22 | 48 |
| Compound | IC50 (µM) Day 2 | IC50 (µM) Day 3 | IC50 (µM) Day 6 |
|---|---|---|---|
| Auranofin | 0.6 | 0.7 | 0.4 |
| GoPI-sugar | 2.8 | 3.9 | 1.7 |
| Compound | Conc. (μM) | % Inhibition of Motility | |||
|---|---|---|---|---|---|
| O. ochengi Adult Female Day 7 | O. ochengi Adult Male Day 5 | O. ochengi mf Day 5 | L. loa mf w/MK2 Cells Day 5 | ||
| Auranofin | 10 | 100 | 100 | 100 | 63 |
| GoPI-sugar | 10 | 51 | 100 | 100 | 38 |
| Compounds | T. b. gambiense IC50 (µM) ± SD | T. b. brucei IC50 (µM) |
|---|---|---|
| Auranofin | 0.21 ± 0.01 | 0.50 |
| GoPI-sugar | 1.11 ± 0.12 | 1.83 |
| Miltefosine 1 | ND | 11.35 |
| Pentamidine 1 | 0.0011 ± 0.0001 | ND |
| Suramin 1 | ND | 0.03 |
| Compounds | Trypanosoma cruzi IC50 (µM) ± SD | Leishmania infantum IC50 (µM) ± SD |
|---|---|---|
| Auranofin | <0.25 | 2.03 ± 0.76 |
| GoPI-sugar | 0.56 ± 0.11 | 2.38 |
| Miltefosine 1 | ND | 11.35 ± 2.88 |
| Benznidazole 1 | 2.89 ± 0.94 | ND |
| Compounds | Leishmania donovani LV9 Axenic Amastigotes IC50 (µM) ± SD | Leishmania Donovani LV9 Intramacrophage Amastigotes IC50 (µm) ± SD |
|---|---|---|
| Auranofin | 0.56 ± 0.03 | 0.70 ± 0.24 |
| GoPI-sugar | 1.45 ± 0.07 | 0.42 ± 0.15 |
| Miltefosine 1 | 1.28 ± 0.12 | 4.49 ± 1.08 |
| Compounds | Acanthamoeba castellanii ATCC 30010 IC50 (µM) ± SD | Cytotoxicity on RAW 264.7 Macrophages CC50 (µM) ± SD |
|---|---|---|
| Auranofin | 5.79 ± 1.02 | 4.43 ± 0.08 |
| GoPI-sugar | 13.04 ± 1.53 | 4.35 ± 0.04 |
| Miltefosine | 9.21 ± 2.04 | >25 |
| Pentamidine | 1.39 ± 0.37 | >25 |
| Compounds | Cytotoxicity on hMRC-5, CC50 (µM) | Cytotoxicity on PMM 1 CC50 (µM) |
|---|---|---|
| Auranofin | 0.52 | 2.00 |
| GoPI-sugar | 0.59 | 8.00 |
| Tamoxifen | 10.63 | ND |
| Miltefosine | ND | 20.00 |
Sample Availability: Samples of the compounds GoPI, GoPI-sugar (LF-028) and of auranofin are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L.; Pomel, S.; Latre de Late, P.; Taravaud, A.; Loiseau, P.M.; Maes, L.; Cho-Ngwa, F.; Bulman, C.A.; Fischer, C.; Sakanari, J.A.; et al. Repurposing Auranofin and Evaluation of a New Gold(I) Compound for the Search of Treatment of Human and Cattle Parasitic Diseases: From Protozoa to Helminth Infections. Molecules 2020, 25, 5075. https://doi.org/10.3390/molecules25215075
Feng L, Pomel S, Latre de Late P, Taravaud A, Loiseau PM, Maes L, Cho-Ngwa F, Bulman CA, Fischer C, Sakanari JA, et al. Repurposing Auranofin and Evaluation of a New Gold(I) Compound for the Search of Treatment of Human and Cattle Parasitic Diseases: From Protozoa to Helminth Infections. Molecules. 2020; 25(21):5075. https://doi.org/10.3390/molecules25215075
Chicago/Turabian StyleFeng, Liwen, Sébastien Pomel, Perle Latre de Late, Alexandre Taravaud, Philippe M. Loiseau, Louis Maes, Fidelis Cho-Ngwa, Christina A. Bulman, Chelsea Fischer, Judy A. Sakanari, and et al. 2020. "Repurposing Auranofin and Evaluation of a New Gold(I) Compound for the Search of Treatment of Human and Cattle Parasitic Diseases: From Protozoa to Helminth Infections" Molecules 25, no. 21: 5075. https://doi.org/10.3390/molecules25215075
APA StyleFeng, L., Pomel, S., Latre de Late, P., Taravaud, A., Loiseau, P. M., Maes, L., Cho-Ngwa, F., Bulman, C. A., Fischer, C., Sakanari, J. A., Ziniel, P. D., Williams, D. L., & Davioud-Charvet, E. (2020). Repurposing Auranofin and Evaluation of a New Gold(I) Compound for the Search of Treatment of Human and Cattle Parasitic Diseases: From Protozoa to Helminth Infections. Molecules, 25(21), 5075. https://doi.org/10.3390/molecules25215075