Influence of Fluorine Substituents on the Electronic Properties of Selenium-N-Heterocyclic Carbene Compounds
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Considerations
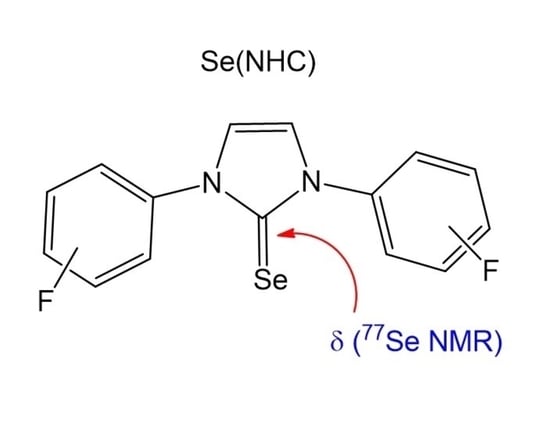
3.1.1. 1,3-Bis(4-fluorophenyl)imidazol-2-selenone, Se(NHC-1)
3.1.2. 1,3-Bis(2,4-difluorophenyl)imidazol-2-selenone, Se(NHC-2)
3.1.3. 1,3-Bis(2,6-difluorophenyl)imidazol-2-selenone, Se(NHC-3)
3.1.4. 1,3-Bis(2,4,5-trifluorophenyl)imidazol-2-selenone, Se(NHC-4)
3.1.5. 1,3-Bis(2,4,6-trifluorophenyl)imidazol-2-selenone, Se(NHC-5)
3.1.6. 1,3-Bis(trimethylphenyl)imidazol-2-selenone, Se(IMes)
3.1.7. 1,3-Bis(phenyl)imidazol-2-selenone, Se(IPh)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vougioukalakis, G.C.; Grubbs, R.H. Ruthenium-based heterocyclic carbene-coordinated olefin metathesis catalysts. Chem. Rev. 2010, 110, 1746–1787. [Google Scholar] [CrossRef] [PubMed]
- Samojłowicz, C.; Bieniek, M.; Grela, K. Ruthenium-based olefin metathesis catalysts bearing N-heterocyclic carbene ligands. Chem. Rev. 2009, 109, 3708–3742. [Google Scholar] [CrossRef] [PubMed]
- Kantchev, E.A.B.; O’Brien, C.J.; Organ, M.G. Palladium complexes of N-heterocyclic carbenes as catalysts for cross-coupling reactions—A synthetic chemist’s perspective. Angew. Chem. Int. Edn. 2007, 46, 2768–2813. [Google Scholar] [CrossRef]
- Fortman, G.C.; Nolan, S.P. N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: A perfect union. Chem. Soc. Rev. 2011, 40, 5151–5169. [Google Scholar] [CrossRef] [PubMed]
- Wuertz, S.; Glorius, F. Surveying sterically demanding N-heterocyclic carbene ligands with restricted flexibility for palladium-catalyzed cross-coupling reactions. Acc. Chem. Res. 2008, 41, 1523–1533. [Google Scholar] [CrossRef]
- Valente, C.; Calimsiz, S.; Hoi, K.H.; Mallik, D.; Sayah, M.; Organ, M.G. The development of bulky palladium NHC complexes for the most-challenging cross-coupling reactions. Angew. Chem. Int. Edn. 2012, 51, 3314–3332. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, L.J.; Wang, W.; Li, S.; Shi, M. Chiral NHC–metal-based asymmetric catalysis. Coord. Chem. Rev. 2012, 256, 804–853. [Google Scholar] [CrossRef]
- Dumas, J.B.; Peligot, E.M. Mémoire sur l’esprit de bois et sur les divers composés éthérés qui en proviennent. Ann. Chim. Phys. 1835, 58, 5–74. [Google Scholar]
- Wanzlick, H.W.; Schönherr, H.J. Direct synthesis of a mercury salt-carbene complex. Angew. Chem. Int. Ed. Engl. 1968, 7, 141–142. [Google Scholar] [CrossRef]
- Öfele, K. 1, 3-Dimethyl-4-imidazolinyliden-(2)-pentacarbonylchrom ein neuer Übergangsmetall-carben-komplex. J. Organomet. Chem. 1968, 12, 42–43. [Google Scholar] [CrossRef]
- Arduengo III, A.J.; Harlow, R.L.; Kline, M. A stable crystalline carbene. J. Am. Chem. Soc. 1991, 113, 361–363. [Google Scholar] [CrossRef]
- Dorta, R.; Stevens, E.D.; Scott, N.M.; Costabile, C.; Cavallo, L.; Hoff, C.D.; Nolan, S.P. Steric and electronic properties of N-heterocyclic carbenes (NHC): A detailed study on their interaction with Ni(CO)4. J. Am. Chem. Soc. 2005, 127, 2485–2495. [Google Scholar] [CrossRef] [PubMed]
- Kelly Iii, R.A.; Clavier, H.; Giudice, S.; Scott, N.M.; Stevens, E.D.; Bordner, J.; Nolan, S.P. Determination of N-heterocyclic carbene (NHC) steric and electronic parameters using the [(NHC)Ir(CO)2Cl] system. Organometallics 2008, 27, 202–210. [Google Scholar] [CrossRef]
- Fantasia, S.; Petersen, J.L.; Jacobsen, H.; Cavallo, L.; Nolan, S.P. Electronic properties of N-heterocyclic carbene (NHC) ligands: Synthetic, structural, and spectroscopic studies of (NHC) Platinum (II) complexes. Organometallics 2007, 26, 5880–5889. [Google Scholar] [CrossRef]
- Back, O.; Henry-Ellinger, M.; Martin, C.D.; Martin, D.; Bertrand, G. 31P NMR Chemical Shifts of Carbene–Phosphinidene Adducts as an Indicator of the π-Accepting Properties of Carbenes. Angew. Chem. Int. Ed. 2013, 52, 2939–2943. [Google Scholar] [CrossRef]
- Liske, A.; Verlinden, K.; Buhl, H.; Schaper, K.; Ganter, C. Determining the π-acceptor properties of N-heterocyclic carbenes by measuring the 77Se NMR chemical shifts of their selenium adducts. Organometallics 2013, 32, 5269–5272. [Google Scholar] [CrossRef]
- Huynh, H.V. Electronic properties of N-heterocyclic carbenes and their experimental determination. Chem. Rev. 2018, 118, 9457–9492. [Google Scholar] [CrossRef]
- Vummaleti, S.V.; Nelson, D.J.; Poater, A.; Gómez-Suárez, A.; Cordes, D.B.; Slawin, A.M.; Nolan, S.P.; Cavallo, L. What can NMR spectroscopy of selenoureas and phosphinidenes teach us about the π-accepting abilities of N-heterocyclic carbenes? Chem. Sci. 2015, 6, 1895–1904. [Google Scholar] [CrossRef] [Green Version]
- Díez-González, S.; Nolan, S.P. Stereoelectronic parameters associated with N-heterocyclic carbene (NHC) ligands: A quest for understanding. Coord. Chem. Rev. 2007, 251, 874–883. [Google Scholar] [CrossRef]
- Jacobsen, H.; Correa, A.; Poater, A.; Costabile, C.; Cavallo, L. Understanding the M (NHC)(NHC= N-heterocyclic carbene) bond. Coord. Chem. Rev. 2009, 253, 687–703. [Google Scholar] [CrossRef]
- Hobbs, M.G.; Knapp, C.J.; Welsh, P.T.; Borau-Garcia, J.; Ziegler, T.; Roesler, R. Anionic N-Heterocyclic Carbenes with N, N′-Bis(fluoroaryl) and N, N′-Bis(perfluoroaryl) Substituents. Chem. Eur. J. 2010, 16, 14520–14533. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, K.; Suresh, P.; Babu, C.N.; Sathyanarayana, A.; Prabusankar, G. Heavier chalcogenone complexes of bismuth (III) trihalides: Potential catalysts for acylative cleavage of cyclic ethers. RSC Adv. 2015, 5, 15579–15590. [Google Scholar] [CrossRef]
- Verlinden, K.; Buhl, H.; Frank, W.; Ganter, C. Determining the Ligand Properties of N-Heterocyclic Carbenes from 77Se NMR Parameters. Eur. J. Inorg. Chem. 2015, 14, 2416–2425. [Google Scholar] [CrossRef]
- Junor, G.P.; Lorkowski, J.; Weinstein, C.M.; Jazzar, R.; Pietraszuk, C.; Bertrand, G. The Influence of C (sp3) H–Selenium Interactions on the 77Se NMR Quantification of the π-Accepting Properties of Carbenes. Angew. Chem. Int. Ed. 2020. [Google Scholar] [CrossRef]
- Jamil, M.S.S.; Alkaabi, S.; Brisdon, A.K. Simple NMR predictors of catalytic hydrogenation activity for [Rh(cod)Cl(NHC)] complexes featuring fluorinated NHC ligands. Dalton Trans. 2019, 48, 9317–9327. [Google Scholar] [CrossRef]
Sample Availability: Not available. |







| Entry | NHC Precursor | Compound | Yield (%) | δ 77Se (ppm) |
|---|---|---|---|---|
| 1 | NHC-1 | Se(NHC-1) | 79 | 60.7 |
| 2 | NHC-2 | Se(NHC-2) | 81 | 66.6 |
| 3 | NHC-3 | Se(NHC-3) | 85 | 68.3 |
| 4 | NHC-4 | Se(NHC-4) | 82 | 67.4 |
| 5 | NHC-5 | Se(NHC-5) | 83 | 73.0 |
| 6 | IMes | Se(IMes) | 81 | 26.7 |
| 7 | IPh | Se(IPh) | 68 | 24.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamil, M.S.S.; Endot, N.A. Influence of Fluorine Substituents on the Electronic Properties of Selenium-N-Heterocyclic Carbene Compounds. Molecules 2020, 25, 5161. https://doi.org/10.3390/molecules25215161
Jamil MSS, Endot NA. Influence of Fluorine Substituents on the Electronic Properties of Selenium-N-Heterocyclic Carbene Compounds. Molecules. 2020; 25(21):5161. https://doi.org/10.3390/molecules25215161
Chicago/Turabian StyleJamil, Mohamad Shazwan Shah, and Nor Azam Endot. 2020. "Influence of Fluorine Substituents on the Electronic Properties of Selenium-N-Heterocyclic Carbene Compounds" Molecules 25, no. 21: 5161. https://doi.org/10.3390/molecules25215161
APA StyleJamil, M. S. S., & Endot, N. A. (2020). Influence of Fluorine Substituents on the Electronic Properties of Selenium-N-Heterocyclic Carbene Compounds. Molecules, 25(21), 5161. https://doi.org/10.3390/molecules25215161