Antibacterial Activity and Pharmacokinetic Profile of a Promising Antibacterial Agent: 22-(2-Amino-phenylsulfanyl)-22-Deoxypleuromutilin
Abstract
:1. Introduction
2. Results
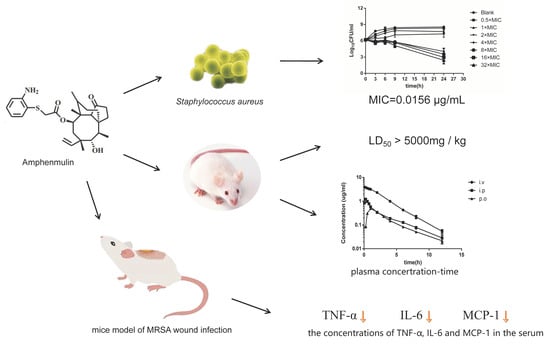
2.1. Biological Evaluation
2.2. Acute Oral Toxicity Study
2.3. Pharmacokinetic Analysis
2.4. The Therapeutic Effect in an Experimental Model of MRSA Wound Infection
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Microorganisms
4.3. Animals
4.4. Biological Evaluation
4.4.1. Minimum Inhibitory Concentration Testing
4.4.2. Constant Concentration Time-Kill Curves
4.4.3. The Postantibiotic Effect
4.5. Acute Toxicity
4.6. Pharmacokinetic Studies
4.7. The Therapeutic Effect of Amphenmulin in an Experimental Model of MRSA Wound Infection
4.7.1. Mice Model of MRSA Wound Infection
4.7.2. Examination of Wound Healing
Determination of Wound Size
Assessment of Wound Infection
Serum Levels of TNF-α, MCP-1 and IL-6
4.7.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Monecke, S.; Coombs, G.; Shore, A.C.; Coleman, D.C.; Akpaka, P.E.; Borg, M.; Chow, H.; Ip, M.; Jatzwauk, L.; Jonas, D.; et al. A field guide to pandemic, epidemic and sporadic clones of methicillin-resistant staphylococcus aureus. PLoS ONE 2011, 6, e17936. [Google Scholar] [CrossRef]
- Watkins, R.R.; Holubar, M.; David, M.Z. Antimicrobial resistance in methicillin-resistant staphylococcus aureus to newer antimicrobial agents. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Nyasulu, P.; Chipolombwe, J.; Török, M.E.; Mbelle, N. Methicillin-resistant Staphylococcus aureus multiple sites surveillance: A systemic review of the literature. Infect. Drug Resist. 2016, 9, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carruthers, N.J.; Stemmer, P.M.; Media, J.; Swartz, K.; Wang, X.; Aube, N.; Hamann, M.T.; Valeriote, F.; Shaw, J. The anti-MRSA compound 3-O-alpha-L-(2″,3″-di-p-coumaroyl)rhamnoside (KCR) inhibits protein synthesis in Staphylococcus aureus. J. Proteom. 2020, 210, 103539. [Google Scholar] [CrossRef] [PubMed]
- Willyard, C. The drug-resistant bacteria that pose the greatest health threats. Nat. News 2017, 543, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/en/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 22 December 2019).
- Kavanagh, F.; Hervey, A.; Robbins, W.J. Antibiotic substances from basidiomycetes VIII. Pleurotus multilus (Fr.) sacc. and pleurotus passeckerianus pilat. Proc. Natl. Acad. Sci. USA 1951, 37, 570–574. [Google Scholar] [CrossRef] [Green Version]
- Davidovich, C.; Bashan, A.; Auerbach-Nevo, T.; Yaggie, R.D.; Gontarek, R.R.; Yonath, A. Induced-Fit tightens pleuromutilins binding to ribosomes and remote interactions enable their selectivity. Proc. Natl. Acad. Sci. USA 2007, 104, 4291–4296. [Google Scholar] [CrossRef] [Green Version]
- Yan, K.; Madden, L.; Choudhry, A.E.; Voigt, C.S.; Copeland, R.A.; Gontarek, R.R. Biochemical characterization of the interactions of the novel pleuromutilin derivative retapamulin with bacterial ribosomes. Antimicrob. Agents Chemother. 2006, 50, 3875–3881. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.-Z.; Liu, Y.-H.; Chen, J.-X. Pleuromutilin and its derivatives-the lead compounds for novel antibiotics. Mini-Rev. Med. Chem. 2012, 12, 53–61. [Google Scholar] [CrossRef]
- Fazakerley, N.J.; Procter, D.J. Synthesis and synthetic chemistry of pleuromutilin. Tetrahedron 2014, 70, 6911–6930. [Google Scholar] [CrossRef] [Green Version]
- Mu, S.; Liu, H.; Zhang, L.; Wang, X.; Xue, F.; Zhang, Y. Synthesis and biological evaluation of novel thioether pleuromutilin derivatives. Boil. Pharm. Bull. 2017, 40, 1165–1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Xiao, S.; Zhang, D.; Mu, S.; Zhang, L.; Wang, X.; Xue, F. Synthesis and antibacterial activity of novel pleuromutilin derivatives. Boil. Pharm. Bull. 2015, 38, 1041–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirokawa, Y.; Kinoshita, H.; Tanaka, T.; Nakamura, T.; Fujimoto, K.; Kashimoto, S.; Kojima, T.; Kato, S. Pleuromutilin derivatives having a purine ring. Part 3: Synthesis and antibacterial activity of novel compounds possessing a piperazine ring spacer. Bioorganic Med. Chem. Lett. 2009, 19, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Jiang, Z.; Cai, Z.; Liu, X.; He, H.; Yang, Y. Water-soluble phosphate prodrugs of pleuromutilin analogues with potent in vivo antibacterial activity against Gram-positive pathogens. Bioorganic Med. Chem. Lett. 2009, 19, 5407–5410. [Google Scholar] [CrossRef]
- Lemieux, M.R.; Siricilla, S.; Mitachi, K.; Eslamimehr, S.; Wang, Y.; Yang, N.; Pressly, J.D.; Kong, Y.; Park, F.; Franzblau, S.G.; et al. An antimycobacterial pleuromutilin analogue effective against dormant bacilli. Bioorganic Med. Chem. 2018, 26, 4787–4796. [Google Scholar] [CrossRef] [PubMed]
- Shang, R.; Pu, X.; Xu, X.; Xin, Z.; Zhang, C.; Guo, W.; Liu, Y.; Liang, J. Synthesis and biological activities of novel pleuromutilin derivatives with a substituted thiadiazole moiety as potent drug-resistant bacteria inhibitors. J. Med. Chem. 2014, 57, 5664–5678. [Google Scholar] [CrossRef] [PubMed]
- Laber, G.; Schütze, E. In vivo efficacy of 81.723 hfu, a new pleuromutilin derivative against experimentally induced airsacculitis in chicks and turkey poults. Antimicrob. Agents Chemother. 1975, 7, 517–521. [Google Scholar] [CrossRef] [Green Version]
- Drews, J.; Georgopoulos, A.; Laber, G.; Schütze, E.; Unger, J.; Tagg, J.R.; Dajani, A.S.; Wannamaker, L.W. Antimicrobial Activities of 81.723 hfu, a New pleuromutilin derivative. Antimicrob. Agents Chemother. 1975, 7, 507–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rittenhouse, S.; Biswas, S.; Broskey, J.; McCloskey, L.; Moore, T.; Vasey, S.; West, J.; Zalacain, M.; Zonis, R.; Payne, D. Selection of retapamulin, a novel pleuromutilin for topical use. Antimicrob. Agents Chemother. 2006, 50, 3882–3885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FDA Approves New Antibiotic to Treat Community-Acquired Bacterial Pneumonia. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-antibiotic-treat-community-acquired-bacterial-pneumonia (accessed on 22 December 2019).
- Zhang, Z.-S.; Huang, Y.-Z.; Luo, J.; Jin, Z.; Liu, Y.-H.; Tang, Y.-Z. Synthesis and antibacterial activities of novel pleuromutilin derivatives bearing an aminothiophenol moiety. Chem. Boil. Drug Des. 2018, 92, 1627–1637. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, X.; Chu, X.; Zhang, X.; Song, K.; Jiang, Y.; Yu, L.; Deng, X. Preventive effects of valnemulin on lipopolysaccharide-induced acute lung injury in mice. Inflammation 2010, 33, 306–314. [Google Scholar] [CrossRef]
- Zhang, Z.-S.; Huang, Y.-Z.; Luo, J.; Wang, B.-F.; Jin, Z.; Liu, Y.-H.; Tang, Y.-Z. Synthesis and antibacterial activity against MRSA of pleuromutilin derivatives possessing a mercaptoethylamine linker. Med. Chem. 2018, 14, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.H.; Yu, Y.; Zhou, Y.F.; Shi, W.; Deng, H.; Liu, Y.H. Postantibiotic effect and postantibiotic sub-minimum inhibitory concentration effect of valnemulin against Staphylococcus aureus isolates from swine and chickens. Lett. Appl. Microbiol. 2014, 58, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Yang, Y.; Chen, J.; Tang, Y. Inhibition of pro-inflammatory mediators in RAW264.7 cells by 7-hydroxyflavone and 7,8-dihydroxyflavone. J. Pharm. Pharmacol. 2017, 69, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Fei, C.; She, R.; Li, G.; Zhang, L.; Fan, W.; Xia, S.; Xue, F. Safety and clinical efficacy of tenvermectin, a novel antiparasitic 16-membered macrocyclic lactone antibiotics. Eur. J. Pharm. Sci. 2018, 117, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; He, L.; Green, J.C.; Blevins, H.; Guo, C.; Patel, S.H.; Halquist, M.S.; McRae, M.; Venitz, J.; Wang, X.-Y.; et al. Discovery of second-generation NLRP3 inflammasome inhibitors: Design, synthesis, and biological characterization. J. Med. Chem. 2019, 62, 9718–9731. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, S.; Csajka, C.; Buclin, T.; Leyvraz, S.; Lejeune, F.; Decosterd, L.A.; Stupp, R. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin. Cancer Res. 2004, 10, 3728–3736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirokawa, Y.; Kinoshita, H.; Tanaka, T.; Nakamura, T.; Fujimoto, K.; Kashimoto, S.; Kojima, T.; Kato, S. Pleuromutilin derivatives having a purine ring. Part 1: New compounds with promising antibacterial activity against resistant Gram-positive pathogens. Bioorganic Med. Chem. Lett. 2008, 18, 3556–3561. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Feng, H.; Xiong, H.; Zhang, L.; Song, Y.; Yu, L.; Deng, X. Valnemulin downregulates nitric oxide, prostaglandin E2, and cytokine production via inhibition of NF-κB and MAPK activity. Int. Immunopharmacol. 2009, 9, 810–816. [Google Scholar] [CrossRef]
- Watkins, R.L.; Pallister, K.B.; Voyich, J.M. The SaeR/S gene regulatory system induces a pro-inflammatory cytokine response during staphylococcus aureus infection. PLoS ONE 2011, 6, e19939. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.C.; Seong, I.-W.; Kim, J.-S.; Cheon, K.-A.; Gu, S.H.; Kim, H.H.; Park, K.H. Enhancement of cutaneous immune response to bacterial infection after low-level light therapy with 1072nm infrared light: A preliminary study. J. Photochem. Photobiol. B: Boil. 2011, 105, 175–182. [Google Scholar]
- Huang, H.-N.; Rajanbabu, V.; Pan, C.-Y.; Chan, Y.-L.; Wu, C.-J.; Chen, J.-Y. Use of the antimicrobial peptide Epinecidin-1 to protect against MRSA infection in mice with skin injuries. Biomaterials 2013, 34, 10319–10327. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.M.; Nakamura, S.; Weiser, J.N. Nod2 sensing of lysozyme-digested peptidoglycan promotes macrophage recruitment and clearance of S. pneumoniae colonization in mice. J. Clin. Investig. 2011, 121, 3666–3676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Wang, Y.; Qin, Y.; He, W.; Benlahrech, A.; Zhang, Q.; Jiang, X.; Lu, Z.; Ji, G.; Zheng, Y. Micheliolide provides protection of mice against Staphylococcus aureus and MRSA infection by down-regulating inflammatory response. Sci. Rep. 2017, 7, 41964. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.-A.; Kruzel, M.L.; Actor, J.K. Immunomodulatory effects of recombinant lactoferrin during MRSA infection. Int. Immunopharmacol. 2014, 20, 157–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, M.-L.; Zeng, J.; Fang, X.; Luo, J.; Jin, Z.; Liu, Y.-H.; Tang, Y.-Z. Design, synthesis and antibacterial evaluation of novel pleuromutilin derivatives possessing piperazine linker. Eur. J. Med. Chem. 2017, 127, 286–295. [Google Scholar] [CrossRef]
- Barbour, A.M.; Schmidt, S.; Zhuang, L.; Rand, K.; Derendorf, H. Application of pharmacokinetic/pharmacodynamic modelling and simulation for the prediction of target attainment of ceftobiprole against meticillin-resistant Staphylococcus aureus using minimum inhibitory concentration and time–kill curve based approaches. Int. J. Antimicrob. Agents 2014, 43, 60–67. [Google Scholar] [CrossRef]
- MacKenzie, F.M.; Gould, I.M. The post-antibiotic effect. J. Antimicrob. Chemother. 1993, 32, 519–537. [Google Scholar] [CrossRef]
- Zhang, C.; Yi, Y.; Chen, J.; Xin, R.; Yang, Z.; Guo, Z.; Liang, J.; Shang, R. In vivo efficacy and toxicity studies of a novel antibacterial Agent: 14-O-[(2-Amino-1,3,4-thiadiazol-5-yl)thioacetyl] mutilin. Molecules 2015, 20, 5299–5312. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.; Wang, L.; Gao, H.; Zhou, Y.-H.; Liu, Y.-H.; Tang, Y.-Z. Design, synthesis and biological evaluation of novel pleuromutilin derivatives possessing acetamine phenyl linker. Eur. J. Med. Chem. 2019, 181, 111594. [Google Scholar] [CrossRef]
- Martinez, L.R.; Han, G.; Chacko, M.; Mihu, M.R.; Jacobson, M.; Gialanella, P.; Friedman, A.J.; Nosanchuk, J.D.; Friedman, J.M. Antimicrobial and healing efficacy of sustained release nitric oxide nanoparticles against staphylococcus aureus skin infection. J. Investig. Dermatol. 2009, 129, 2463–2469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds are available from the authors. |







| Compounds | S. aureus ATCC 29213 | E. faecalis ATCC 29212 | E. faecium ATCC 35667 | E. coli ATCC 25922 | S. typhimurium ATCC 14028 | N7 | N9 | N20 | N30 | N54 |
|---|---|---|---|---|---|---|---|---|---|---|
| Amphenmulin | 0.0156 | >128 | 0.125 | >128 | >128 | 4 | 0.0156 | 4 | 8 | 0.0156 |
| Tiamulin | 0.5 | >128 | 2 | >128 | >128 | 16 | 0.5 | 32 | 32 | 0.5 |
| Compounds | Concentrations | PAE (h) | |
|---|---|---|---|
| Exposure for 1 h | Exposure for 2 h | ||
| Amphenmulin | 1× MIC | 0.71 | 1.35 |
| 4× MIC | 1.78 | 2.83 | |
| Parameters | Intravenous Administration | Intraperitoneal Administration | Oral Administration |
|---|---|---|---|
| Cmax (µg/mL) | - | 1.34 ± 0.42 | 0.53 ± 0.18 |
| Tmax (h) | - | 0.32 ± 0.21 | 1.37 ± 0.74 |
| T1/2 (h) | 1.92 ± 0.28 | 2.64 ± 0.72 | 2.91 ± 0.81 |
| AUC0→∞(µg·h/mL) | 12.23 ± 1.35 | 2.52 ± 0.81 | 1.67 ± 0.66 |
| MRT (h) | 2.57 ± 0.19 | 3.28 ± 0.42 | 4.01 ± 0.62 |
| CLβ (L/h/kg) | 0.82 ± 0.09 | - | - |
| CLβ/F (L/h/kg) | - | 4.08 ± 1.14 | 6.31 ± 2.26 |
| Vz (L/kg) | 2.17 ± 0.42 | - | - |
| Vz/F (L/kg) | - | 15.92 ± 7.41 | 28.46 ± 9.01 |
| F% | - | 20.71% | 13.65% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, X.; Fang, X.; Zhang, Z.; Jin, Z.; Xi, G.; Liu, Y.; Tang, Y. Antibacterial Activity and Pharmacokinetic Profile of a Promising Antibacterial Agent: 22-(2-Amino-phenylsulfanyl)-22-Deoxypleuromutilin. Molecules 2020, 25, 878. https://doi.org/10.3390/molecules25040878
Zuo X, Fang X, Zhang Z, Jin Z, Xi G, Liu Y, Tang Y. Antibacterial Activity and Pharmacokinetic Profile of a Promising Antibacterial Agent: 22-(2-Amino-phenylsulfanyl)-22-Deoxypleuromutilin. Molecules. 2020; 25(4):878. https://doi.org/10.3390/molecules25040878
Chicago/Turabian StyleZuo, Xiangyi, Xi Fang, Zhaosheng Zhang, Zhen Jin, Gaolei Xi, Yahong Liu, and Youzhi Tang. 2020. "Antibacterial Activity and Pharmacokinetic Profile of a Promising Antibacterial Agent: 22-(2-Amino-phenylsulfanyl)-22-Deoxypleuromutilin" Molecules 25, no. 4: 878. https://doi.org/10.3390/molecules25040878
APA StyleZuo, X., Fang, X., Zhang, Z., Jin, Z., Xi, G., Liu, Y., & Tang, Y. (2020). Antibacterial Activity and Pharmacokinetic Profile of a Promising Antibacterial Agent: 22-(2-Amino-phenylsulfanyl)-22-Deoxypleuromutilin. Molecules, 25(4), 878. https://doi.org/10.3390/molecules25040878