Advanced Research on the Antioxidant Activity and Mechanism of Polyphenols from Hippophae Species—A Review
Abstract
:1. Introduction
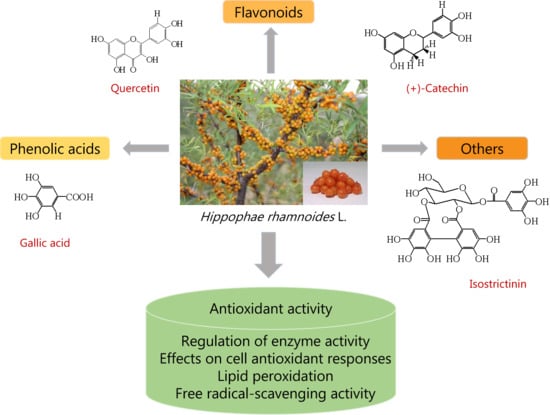
2. Chemical Constituents and Structures of Polyphenols
2.1. Flavonoids
2.2. Phenolic Acids
2.3. Others
3. Antioxidant Activity
3.1. Regulation of Enzyme Activity
3.1.1. Enhancing Antioxidant Enzyme Activity
3.1.2. Inhibiting the Production of Oxidase
3.2. Effects on Cell Antioxidant Responses
3.3. Others
4. Application
4.1. Medicinal Values of Hippophae Species
4.2. Food Values of Hippophae Species
4.3. Cosmetic Values of Hippophae Species
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Yang, L.B.; Jia, M.; Liu, S.J.; Diao, Y.B.; Zhang, Y.Q.; Xiao, Q.R. Anti-oxidation effect of the Shaji Tablet on mice. Hebei Med. J. 2015, 37, 649–651. [Google Scholar] [CrossRef]
- Saikia, M.; Handique, P.J. Antioxidant and antibacterial activity of leaf, bark, pulp and seed extracts of seabuckthorn (Hippophae salicifolia D. Don) of Sikkim Himalayas. J. Med. Plants Res. 2013, 7, 1330–1338. [Google Scholar]
- Liu, Y.; Sun, W.; Liu, C.; Zhang, Y.Q.; Chen, Y.L.; Song, M.; Fan, G.; Liu, X.; Xiang, L.; Zhang, Y. Identifcation of Hippophae species (Shaji) through DNA barcodes. Chin. Med. 2015, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.J. Effect of Seabuckthorn Protein on Lipid Metabolism and Intestinal Microbial Community in Streptozotocin-Induced Diabetic Mice. Master’s Thesis, Hefei University of Technology, Hefei, China, 2017. [Google Scholar] [CrossRef]
- Lu, R.S. Research on seabuckthorn (Hippophae L.) resources in China. Acta. Hortic. Sin. 1990, 17, 177–183, 248. [Google Scholar]
- Du, J.; Xi, Y.L.; Song, C.M. Effect of Sea Buckthorn Powder on Hepatic Lipid Metabolism and Oxidative Stress in Rats. Mod. Food Sci. Technol. 2017, 33, 8–12, 133. [Google Scholar] [CrossRef]
- Di Mauro, M.D.; Giardina, R.C.; Fava, G.; Mirabella, E.F.; Acquaviva, R.; Renis, M.; D’Antona, N. Polyphenolic profile and antioxidant activity of olive mill wastewater from two Sicilian olive cultivars: Cerasuola and Nocellara etnea. Eur. Food Res. Technol. 2017, 243, 1895–1903. [Google Scholar] [CrossRef]
- Di Mauro, M.D.; Fava, G.; Spampinato, M.; Aleo, D.; Melilli, B.; Saita, M.G.; Centonze, G.; Maggiore, R.; D’Antona, N. Polyphenolic fraction from olive mill wastewater: Scale-up and in vitro studies for ophthalmic nutraceutical applications. Antioxidants 2019, 8, 462. [Google Scholar] [CrossRef] [Green Version]
- Acquaviva, R.; Sorrenti, V.; Santangelo, R.; Cardile, V.; Tomasello, B.; Malfa, G.; Vanella, L.; Amodeo, A.; Genovese, C.; Mastrojeni, S.; et al. Effects of an extract of Celtis aetnensis (Tornab.) Strobl twigs on human colon cancer cell cultures. Oncol. Rep. 2016, 36, 2298–2304. [Google Scholar] [CrossRef]
- Wani, T.A.; Wani, S.M.; Ahmad, M.; Gani1, A.; Masoodi1, F.A. Bioactive profile, health benefits and safety evaluation of sea buckthorn (Hippophae rhamnoides L.): A review. Cogent. Food. Agric. 2016, 2, 1128519. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition and antioxidant potential of grain legume seeds: A review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.S.; Wan, L. Types of bioactivity substances of genus Hippophae L. and their functions in physiology and pharmacology. Hippophae 2007, 20, 1–12. [Google Scholar]
- Du, L.L.; Chen, W.; Li, Y.Z.; Wang, X.Y. Determination of the polyphenols in berries of Hippophae rhamnoides subsp. wolongensis. West. Chin. J. Pharm. Sci. 2015, 30, 336–338. [Google Scholar] [CrossRef]
- Li, J.X.; Wang, B.Y. Folin-ciocalteu colorimetric determination of total polyphenols in mulberry fruits. Food Sci. 2009, 30, 292–295. [Google Scholar] [CrossRef]
- Fredes, C.; Montenegro, G.; Zoffoli, J.P.; Santander, F.; Robert, P. Comparison of the total phenolic content, total anthocyanin content and antioxidant activity of polyphenol-rich fruits grown in Chile. Cienc. Investig. Agrar. 2014, 41, 9–10. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.; Cho, E.; Jung, H.; Yi, C.H.; Lee, B.; Hwang, K.T. Antioxidant activities of sea buckthorn leaf tea extracts compared with green tea extracts. Food Sci. Biotechnol. 2014, 23, 1295–1303. [Google Scholar] [CrossRef]
- Lv, Q.; Tu, Y.H.; Li, J.; Liu, H.T.; Zhang, R.Q.; Luo, Y.; Kang, Y.Q. Comparative Study on Content of Tea Polyphenols Extracted from Different Tea Plant Tissue. J. Guiyang Med. Coll. 2016, 41, 265–267. [Google Scholar] [CrossRef]
- Zu, Y.; Li, C.; Fu, Y.J.; Zhao, C.J. Simultaneous determination of catechin, rutin, quercetin kaempferol and isorhamnetin in the extract of sea buckthorn (Hippophae rhamnoides L.) leaves by RP-HPLC with DAD. J. Pharm. Biomed. Anal 2006, 41, 714–719. [Google Scholar] [CrossRef]
- Cao, F.; Chen, G.R.; Wang, A.G. Study on Hippophae rhamnoides flavonoids and their physiological functions. Beverage Ind. 2003, 6, 5–9. [Google Scholar] [CrossRef]
- Xing, J.X. Comparative Study on Total Flavonoids Content in Different Parts of Hippophae Rhamnoides Linn. Shanxi Forestry Sci. Technol. 2018, 47, 4–5. [Google Scholar]
- Fu, G.X.; Feng, R.Z.; Xiao, P.G. Determination and Comparison of Total Flavonoids in Seabuckthorn Leaves of Different Species and Different Harvesting Times. J. Tradit. Chin. Med. 1997, 22, 147–148. [Google Scholar] [CrossRef]
- RiSch, D.; Krumbein, A.; Kroh, L.W. Antioxidant gallocatechins, dimeric and trimeric proanthocyanidins from sea buckthorn (Hippophae rhamnoides) pomace. Eur. Food Res. Technol. 2004, 219, 605–613. [Google Scholar] [CrossRef]
- Panossian, A.; Wagner, H. From Traditional to Evidence-Based Use of Hippophae rhamnoides L.: Chemical Composition, Experimental, and Clinical Pharmacology of Sea Buckthorn Berries and Leaves Extracts. Evidence and Rational Based Research on Chinese Drugs; Wagner, H., Merzenich, G.U., Eds.; Springer-Verlag: Wien, Austria; London, UK; New York, NY, USA, 2013; Volume 5, pp. 181–236. [Google Scholar] [CrossRef]
- Qin, Z.X.; Yu, Z.; Qi, M.D.; Zhang, Q.; Liu, S.; Li, M.H.; Liu, Y.G.; Liu, Y. Rapid analysis of compounds in leaves of Chinese seabuckthorn and Tibetan seabuckthorn by UPLC/Q-TOF-MS. Chin. J. Tradit. Chin. Med. 2016, 41, 1461–1468. [Google Scholar] [CrossRef]
- Ran, B.B.; Li, W.D. Research progress on chemical constituents and their differences between seabuckthorn berries and leaves. Chin. J. Tradit. Chin. Med. 2019, 44, 1767–1773. [Google Scholar]
- Kumar, M.S.; Dutta, R.; Prasad, D.; Misra, K. Subcritical water extraction of antioxidant compounds from Seabuckthorn (Hippophae rhamnoides) leaves for the comparative evaluation of antioxidant activity. Food Chem. 2011, 127, 1309–1316. [Google Scholar] [CrossRef]
- Zhang, S.M. Exttraction, Isolation and Identification of Flavones in Seabuckthorn (Hippophae rhamnoides L.) Fruits. Master’s Thesis, Jilin Agricultural University, Jilin, China, 2004. [Google Scholar]
- Chen, C.; Zhang, H.; Gu, H.; Yin, H.X.; Chen, Y. Flavonoid glycosides from Hippophae rhamnoides subsp. sinensis Rousi. West Chin. J. Pharmaceut. Sci. 2007, 22, 367–370. [Google Scholar]
- Guliyev, V.B.; Gul, M.; Yildirim, A. Hippophae rhamnoides L.: Chromatographic methods to determine chemical composition, use in traditional medicine and pharmacological effects. J. Chromatogr. B 2004, 812, 291–307. [Google Scholar] [CrossRef]
- Chen, C. Resources and Quality Evaluation of Genus Hippophae on Qinghai-Tibet Plateau. Ph.D. Thesis, Sichuan University, Chengdu, China, 2007. [Google Scholar]
- Kim, J.S.; Kwon, Y.S.; Sa, Y.J.; Kim, M.J. Isolation and identification of sea buckthorn (Hippophae rhamnoides) phenolics with antioxidant activity and α-glucosidase inhibitory effect. J. Agric. Food Chem. 2011, 59, 138–144. [Google Scholar] [CrossRef]
- Chen, C.; Xu, X.M.; Chen, Y.; Yu, M.Y.; Wen, F.Y.; Zhang, H. Identification, quantification and antioxidant activity of acylated flavonol glycosides from sea buckthorn (Hippophae rhamnoides ssp. sinensis). Food Chem. 2013, 141, 1573–1579. [Google Scholar] [CrossRef]
- Zhao, L.P.; Zhang, N.Z.; Xu, F.C. Study on Chemical Constituents of Hippophae thibetana. Anhui Agric. Sci. 2016, 44, 75–76. [Google Scholar] [CrossRef]
- Liu, Y.; Lian, Y.S.; Wang, Y.L.; Li, M.H.; Xiao, P.G. Review of seabuckthorn research and development and its significance. Chin. J. Tradit. Chin. Med. 2014, 39, 1547–1552. [Google Scholar] [CrossRef]
- Rosch, D.; Mugge, C.V.; Kroh, L.W. Antioxidant oligomeric proanthocyanidins from sea buckthorn (Hippophae rhamnoides) Pomace. J. Agric. Food Chem. 2004, 52, 6712–6718. [Google Scholar] [CrossRef] [PubMed]
- Ruan, D.L.; Tao, H.R.; Li, H. Isolation and Identification of 4,4’-Diol-2’-methoxychalcone from Leaves of Seabuckthorn. Hippophae 2004, 17, 24–27. [Google Scholar]
- Arimboor, R.; Kumar, K.S.; Arumughan, C. Simultaneous estimation of phenolic acids in sea buckthorn (Hippophae rhamnoides) using RP-HPLC with DAD. J. Pharmaceut. Biomed. Anal. 2008, 47, 31–38. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; Wu, F.H. Flavonoids in Hippophae rhamnoides and Their Medicinal Value. Hippophae 1997, 39–41. [Google Scholar]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Sheichenko, O.P.; Sheichenko, V.I.; Fadeeva, I.I.; Zolotarev, B.M.; Tolkachev, O.N. Tannins from leaves of Hippophae rhamnoides. Chem. Nat. Comp. 1987, 23, 756–760. [Google Scholar] [CrossRef]
- Yoshida, T.; Tanaka, K.; Chen, X.M.; Okuda, T. Tannins from Hippophae rhamnoides. Phytochemistry 1991, 30, 663–666. [Google Scholar] [CrossRef]
- Zhang, J.W.; Wang, Y.; Xu, L.M.; Ma, X.L.; Sun, Y.X.; Jiang, W.P. Determination of gossypol in Hippophae rhamnoides L. seed oil. J. Nat. Sci. Jilin Univ. 1996, 3, 105–106. [Google Scholar]
- McAuley, C.Y. Phenolic Compounds in Mentha spicata: Quantification, Identification and Antioxidant Activity. Master’s Thesis, The University of Guelph, Guelph, ON, Canada, 2002. [Google Scholar]
- Tan, P.X.; Ye, T.; Liu, X.X.; He, J.H. Research Advances in Antioxidant Composition of Botanical Extracts and Their Action Mechanisms. Food Sci. 2010, 31, 288–292. [Google Scholar]
- Lu, X.X. Research Progress in Antioxidant Mechanism of Flavnonids. Food Res. Dev. 2012, 33, 220–224. [Google Scholar] [CrossRef]
- Wang, L.X.; Xie, Y.H.; Zhang, G.G. Application of Phytogenic Antioxidants and Its Mechanisms. J. Anim. Nut. 2017, 29, 1481–1488. [Google Scholar] [CrossRef]
- Maheshwari, D.T.; Yogendra Kumar, M.S.; Verma, S.K.; Singh, V.K.; Singh, S.N. Antioxidant and hepatoprotective activities of phenolic rich fraction of Seabuckthorn (Hippophae rhamnoides L.) leaves. Food Chem. Toxicol. 2011, 49, 2422–2428. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, F.H.; Zhao, L.; Zhang, D.; Wang, O.; Guo, X.X.; Lu, F.; Yang, X.; Ji, B.P.; Deng, Q.C. Protective Effect of Total Flavones from Hippophae rhamnoides L. against Visible Light-Induced Retinal Degeneration in Pigmented Rabbits. J. Agric. Food Chem. 2016, 64, 161–170. [Google Scholar] [CrossRef]
- Zhang, J.C.; Wang, C.T.; Liu, Y.; Shi, X.Q.; Zhao, D.; Li, M.; Wang, C.T.; Sun, B.G. Anti-Aging Effect and Its Mechanism of Ethanol Extract from Sea Buckthorn Seed Meal in Caenorhabditis elegans. Food Sci. 2017, 38, 141–148. [Google Scholar] [CrossRef]
- Reid, A.B.; Kurten, R.C.; McCullough, S.S.; Brock, R.W.; Hinson, J.A. Mechanisms of acetaminophen-induced hepatotoxicity: Role of oxidative stress and mitochondrial permeability transition in freshly isolated mouse hepatocytes. J. Pharmacol. Exp. Ther. 2005, 312, 509–516. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Rehman, H.; Wright, G.L.; Zhong, Z. Inhibition of inducible nitric oxide synthase prevents mitochondrial damage and improves survival of steatotic partial liver grafts. Transplantation 2010, 89, 291–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.G.; Li, H.R.; Wang, L.Y.; Li, Y.H.; Lu, S.G.; Wen, X.F.; Wang, J.; Daikonya, A.; Kitanaka, S. Triterpenoids from Hippophae rhamnoides L. and their nitric oxide production-inhibitory and DPPH radical-scavenging activities. Chem. Pharm. Bull. 2007, 55, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, S.; Ruma, D.; Gitika, B.; Sharma, S.K.; Pauline, T.; Sai Ram, M.; Ilavazhagan, G.; Swahney, R.C.; Kumar, D.; Banerjee, P.K. Antioxidant activities of seabuckthorn (Hippophae rhamnoides) during hypoxia induced oxidative stress in glial cells. Mol. Cellul. Biochem. 2005, 278, 9–14. [Google Scholar] [CrossRef]
- Bai, H.M. Study of Seabuckthorn Flavonoids on Antioxidation and Immune Adjustment Function of Rats. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2009. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, L.; Li, H.; Cui, W.W.; Ren, Z.Y. The Research of Anti-oxidativ Function of Quercetin. Rain Fed. Crops. 2009, 29, 99–100. [Google Scholar] [CrossRef]
- Li, X. Antiradical effects of tea polyphenols. Sci. Technol. Vision. 2014, 352–353. [Google Scholar] [CrossRef]
- Zou, Y.; Lu, Y.; Wei, D. Antioxidant Activity of a Flavonoid-Rich Extract of Hypericum perforatum L. In Vitro. J. Agric. Food Chem. 2004, 52, 5032–5039. [Google Scholar] [CrossRef]
- Upadhyay, N.K.; Kumar, M.S.Y.; Gupta, A. Antioxidant, cytoprotective and antibacterial effects of Sea buckthorn (Hippophae rhamnoides L.) leaves. Food Chem. Toxicol. 2010, 48, 3443–3448. [Google Scholar] [CrossRef] [PubMed]
- Ting, H.C.; Hsu, Y.W.; Tsai, C.F.; Lu, F.J.; Chou, M.C.; Chen, W.K. The in vitro and in vivo antioxidant properties of seabuckthorn (Hippophae rhamnoides L.) seed oil. Food Chem. 2011, 125, 652–659. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Advantages and limitations of common testing methods for antioxidants. Free Radical Res. 2015, 49, 633–649. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Qi, L.N.; Jiang, J.L.; Zhang, J.F.; Zhang, L.L.; Wang, T. Curcumin Protects Human Trophoblast HTR8/SVneo Cells from H2O2-Induced Oxidative Stress by Activating Nrf2 Signaling Pathway. Antioxidants 2020, 9, 121. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Ma, H.P.; Qi, X.Z.; Meng, P.; Jia, Z.P. Progression of Nrf2-ARE Signaling Pathway in Protection of Oxidative Stress Injury of the Body. Med. Pharm. J. Chin. People’s Liberation Army. 2015, 27, 21–27. [Google Scholar] [CrossRef]
- Thimmulappa, R.K.; Mai, K.H.; Srisuma, S.; Kensler, T.W. Identification of Nrf2-regulated Genes Induced by the Chemopreventive Agent Sulforaphane by Oligonucleotide Microarray. Cancer Res. 2002, 62, 196–203. [Google Scholar] [CrossRef]
- Kwak, M.K.; Akabayashi, N.; Itoh, K.; Motohash, H.; Yamamoto, M.; Kensler, T.W. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway: Identification of novel gene clusters for cell survival. Biol. Chem. 2003, 278, 8135–8145. [Google Scholar] [CrossRef] [Green Version]
- Molagoda, I.M.N.; Lee, K.T.; Choi, Y.H.; Kim, G. Anthocyanins from Hibiscus syriacus L. Inhibit Oxidative Stress-mediated Apoptosis by Activating the Nrf2/HO-1 Signaling Pathway. Antioxidants 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podder, B.; Kim, Y.S.; Song, H.Y. Cytoprotective effect of bioactive sea buckthorn extract on paraquat-exposed A549 cells via induction of Nrf2 and its downstream genes. Mol. Med. Rep. 2013, 8, 1852–1860. [Google Scholar] [CrossRef] [PubMed]
- Zerin, T.; Kim, Y.S.; Hong, S.Y.; Song, H.Y. Protective effect of methylprednisolone on paraquat-induced A549 cell cytotoxicity via induction of efflux transporter, P glycoprotein expression. Toxicol. Lett. 2012, 208, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Na, H.K.; Surh, Y.J. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem. Toxicol. 2008, 46, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.K.; Itoh, K.; Yamamoto, M.; Kensler, T.W. Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: Role of antioxidant response element-like sequences in the nrf2 promoter. Mol. Cell. Biochem. 2002, 22, 2883–2892. [Google Scholar] [CrossRef] [Green Version]
- Han, X.Q.; Zhang, C.X.; Zhang, W.J.; Xiang, B.Y.; Zhang, J.X. Effects of supplementation of different sea buckthorn pomace levels on antioxidation in testis and epi-didymis of ram. J. Shanxi Agric. Univ. (Nat. Sci. Edit.) 2017, 37, 733–742. [Google Scholar] [CrossRef]
- Zhang, L.; Wan, N. Advances in the research of sport fatigue caused by the action of free radical lipid oxidation. Chin. J. Lab. Diagn. 2006, 1104–1108. [Google Scholar] [CrossRef]
- Zhao, H.X.; Zhang, H.S.; Yang, S.F. Phenolic compounds and its antioxidant activities in ethanolic extracts from seven cultivars of Chinese jujube. Food Sci. Hum. Wellness 2014, 3, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Zadernowski, R.; Naczk, M.; Nowak-Polakowska, H.; Nesterowicz, J. Effect of sea buckthorn (Hippophae rhamnoides L.) berry extracts on the activity of lipase and lipoxygenase. J. Food Lipids 2012, 9, 249–258. [Google Scholar] [CrossRef]
- Rop, O.; Ercisli, S.; Mlcek, J.; Jurikova, T.; Hoza, I. Antioxidant and radical scavenging activities in fruits of 6 sea buckthorn (Hippophae rhamnoides L.) cultivars. Turk. J. Agric. For. 2014, 38, 224–232. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, B.W.; Wang, Y.; Kang, J.; Wang, H.Y. Protective effect of the on myocardial ischemia and reperfusion injury in rats. Chin. Pharm. Bull. 1997, 1, 59–61. [Google Scholar]
- Liu, Z.T.; Huang, J.; Wang, Y.X.; An, W.Q. Experiment effect of buckthron extract on lipofusion of cerebral tissues in wistar elderly rats. Chin. J. Gerontol. 2001, 21, 300–301. [Google Scholar]
- Chen, R.; Wang, X.L.; Xu, H.H.; Sun, L.; Yu, Z.L. A Review on Antioxidant Activity of Antioxidants. J. Heze Univ. 2013, 35, 44–49. [Google Scholar] [CrossRef]
- Li, F.H.; Guo, X.H.; Xia, C.Y.; Chen, L.; Ling, B.; Ming, J. Research Advance in Antioxidant Activity of Phenolic Compounds in Whole Grains. Food Sci. 2012, 33, 299–304. [Google Scholar]
- Su, H.L.; Wei, J.; Bi, Y.; Li, J.X.; Yury, Z.; Alexander, K. Antioxidant activity of ethanol extracts from Chinese seabuckthorn berries in vitro and on HepG2 cells. Sci. Technol. Food Ind. 2017, 5, 51–55. [Google Scholar]
- Varshneya, C.; Kant, V.; Mehta, M. Total phenolic contents and free radical scavenging activities of different extracts of seabuckthorn (Hippophae rhamnoides) pomace without seeds. Int. J. Food Sci. Nutr. 2012, 63, 153–159. [Google Scholar] [CrossRef]
- Forman, H.J.; Davies, K.J.A.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radical Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Haleagrahara, N.; Chakravarthi, S.; Kulur, A.B.; Tan, M.Y. Plant flavone apigenin protects against cyclosporine-induced histological and biochemical changes in the kidney in rats. Biomed. Prev. Nutr. 2014, 4, 589–593. [Google Scholar] [CrossRef]
- Coskun, O.; Kanter, M.; Korkmaz, A.; Oter, S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacol. Res. 2005, 51, 117–123. [Google Scholar] [CrossRef]
- Seo, K.; Yang, J.H.; Kim, S.C.; Ku, S.K.; Ki, S.H.; Shin, S.M. The Antioxidant Effects of Isorhamnetin Contribute to Inhibit COX-2 Expression in Response to Inflammation: A Potential Role of HO-1. Inflammation 2014, 37, 712–722. [Google Scholar] [CrossRef]
- Mingjie, Z.; Huanhuan, R.; Jichun, H.; Wang, W.J.; Zheng, Q.S.; Wang, D. Protective Effects of Kaempferol against Myocardial Ischemia/Reperfusion Injury in Isolated Rat Heart via Antioxidant Activity and Inhibition of Glycogen Synthase Kinase-3β. Oxid. Med. Cell Longev. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Barzegar, A. Antioxidant activity of polyphenolic myricetin in vitro cellfree and cell-based systems. Mol. Biol. Res. Commun. 2016, 5, 87–95. [Google Scholar] [PubMed]
- Jayaraman, J.; Veerappan, M.; Namasivayam, N. Potential beneficial effect of naringenin on lipid peroxidation and antioxidant status in rats with ethanol-induced hepatotoxicity. J. Pharm. Pharmacol. 2010, 61, 1383–1390. [Google Scholar] [CrossRef]
- Rajadurai, M.; Prince, P.S.M. Preventive effect of naringin on lipid peroxides and antioxidants in isoproterenol-induced cardiotoxicity in Wistar rats: Biochemical and histopathological evidences. Toxicology 2006, 228, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Kang, K.A.; Zhang, R.; Ko, D.O.; Wang, Z.H.; You, H.J.; Kim, H.S.; Kim, J.S.; Kang, S.S.; Hyun, J.W. Hyperoside prevents oxidative damage induced by hydrogen peroxide in lung fibroblast cells via an antioxidant effect. BBA Gen. Subjects 2008, 1780, 0–1457. [Google Scholar] [CrossRef]
- Kalender, Y.; Kaya, S.; Durak, D.; Uzun, F.G.; Demir, F. Protective effects of catechin and quercetin on antioxidant status, lipid peroxidation and testis-histoarchitecture induced by chlorpyrifos in male rats. Environ. Toxicol. Pharmacol. 2012, 33, 0–148. [Google Scholar] [CrossRef]
- Kamalakkannan, N.; Prince, P.S.M. Rutin improves the antioxidant status in streptozotocin-induced diabetic rat tissues. Mol. Cell Biochem. 2006, 293, 211–219. [Google Scholar] [CrossRef]
- Punithavathi, V.R.; Prince, P.S.M.; Kumar, R.; Selvakumari, J. Antihyperglycaemic, antilipid peroxidative and antioxidant effects of gallic acid on streptozotocin induced diabetic Wistar rats. Eur. J. Pharmacol. 2011, 650, 465–471. [Google Scholar] [CrossRef]
- Ramachandran, V.; Saravanan, R.; Raja, B. Attenuation of oxidative stress by syringic acid on acetaminophen-induced nephrotoxic rats. Comp. Clin. Pathol. 2012, 21, 1559–1564. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, L.; Ruan, Z.; Mi, S.; Jiang, M.; Li, X.L.; Wu, X.; Deng, Z.Y.; Yin, Y.L. Chlorogenic acid ameliorates intestinal mitochondrial injury by increasing antioxidant effects and activity of respiratory complexes. Biosci. Biotechnol. Biochem. 2016, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghaisas, M.M.; Kshirsagar, S.B.; Sahane, R.S. Evaluation of wound healing activity of ferulic acid in diabetic rats. Int. Wound J. 2012, 11, 523–532. [Google Scholar] [CrossRef]
- Qi, D.H.; Liu, H.C.; Liu, Q.J. Development of seabuckthorn healthy fruit nectar. Hippophae 2001, 14, 22–24. [Google Scholar]
- Guo, H.L. Letnod of Fermented Hippophae Composition for Improving Intestinal Immunity. Patent CN108208793A, 29 June 2018. [Google Scholar]
- Li, Y. Liver-Protective Natural Product and Its Preparation Method. Patent CN103417752A, 5 August 2009. [Google Scholar]
- Xiong, B.Q.; Yu, D.; Yuan, J.; Zeng, M.; Zhang, Y.; Du, J.B. The Wild Plant Resources and Utilization of Hippophae in China. Chin. Wild Plant Res. 2004, 23, 25–26. [Google Scholar]
- Zou, W. Rich in Antioxidant Nutrient for Sea Buckthorn Fruit Peel Powder Complex Nutritional Meal Powder and Preparation Method Thereof. Patent CN108783292A, 13 November 2018. [Google Scholar]
- Yan, J. A Kind of Sea-Buckthorn Health-Promoting Edible Salt and Preparation Method Thereof. Patent CN108283306A, 17 July 2018. [Google Scholar]
- Smida, I.; Pentelescu, C.; Pentelescu, O.; Sweidan, A.; Oliviero, N.; Meuric, V.; Martin, B.; Colceriu, L.; BonnaureMallet, M.; Tamanai-Shacoori, Z. Benefits of sea buckthorn (Hippophae rhamnoides) pulp oil-based mouthwash on oral health. J. Appl. Microbiol. 2019, 126, 1594–1605. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.H.; Zhao, Y.Z. Folk prescription of Russian seabuckthorn skin care products. Hippophae 1997, 10, 39–41. [Google Scholar]
- Ren, M.; Gao, X.M. Limitation and Prospect of Animal experiment in the Research of traditional Chinese Medicine. Liaoning J. Tradit. Chin. Med. 2004, 31, 820–821. [Google Scholar] [CrossRef]








| No. | Compounds | Skeletons | R1 | R2 | R3 | R4 | R5 | R6 | Species | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Astragalin | A | Glu | H | H | OH | H | - | H. rhamnoides L. | [23] |
| 2 | Rutin | A | Rut | H | H | OH | OH | - | H. rhamnoides L. subsp. sinensis, H. tibetana S., H. rhamnoides L. | [24,25] |
| 3 | Quercetin | A | H | H | H | OH | OH | - | H. rhamnoides L. subsp. Wolongensis | [14] |
| 4 | Quercetin-3-methyl ether | A | Me | H | H | OH | H | - | H. rhamnoides L. | [26] |
| 5 | Quercetin-3-galactoside (Hyperoside) | A | Gal | H | H | OH | OH | - | H. rhamnoides L. | [27] |
| 6 | Quercetin-3-O-β-d-rutinoside | A | Rut | H | H | OH | OH | - | H. rhamnoides L. subsp. mongolica | [28,29] |
| 7 | Quercetin-3-galactoglucoside | A | Gal | H | - | H | OH | - | H. rhamnoides L. subsp. sinensis | [28] |
| 8 | Quercetin-7-O-glucoside | A | H | Glu | H | OH | OH | - | H. rhamnoides L. subsp. sinensis, H. tibetana S. | [25,26,30] |
| 9 | Quercetin-7-O-α-l-rhamnoside | A | H | Rha | H | OH | OH | - | H. rhamnoides L. subsp. sinensis, H. tibetana S. | [25] |
| 10 | Quercetin-3-O-β-d-glucoside-7-O-α-L-rhamnoside | A | Glu | Rha | H | OH | OH | - | H. rhamnoides L. subsp. sinensis | [31] |
| 11 | Quercetin-3-O-β-d-glucopyranosyl-7-O-α-L-rhamnopyranoside | A | Glu | Rha | H | OH | OH | - | H. rhamnoides L. | [23] |
| 12 | Quercetin-3-O-rhamnoside | A | Rha | H | H | OH | OH | - | H. rhamnoides L. subsp. sinensis, H. tibetana S. | [32] |
| 13 | Quercetin-3-O-β-d-sophoroside-7-O-α-l-rhamnoside | A | Sop | Rha | H | OH | OH | - | H. rhamnoides L. subsp. sinensis, H. tibetana S. | [25] |
| 14 | Quercetin-3-O-glucoside | A | Glu | H | H | OH | OH | - | H. rhamnoides L. subsp. sinensis | [29] |
| 15 | Quercetin-3-O-β-d- Rutinoside-7-O-α-l-glucoside | A | Rut | Glu | H | OH | OH | - | H. tibetana S. | [25] |
| 16 | Quercetin-3-methyl ether | A | OCH3 | H | H | OH | H | - | H. rhamoides L. | [26] |
| 17 | Quercetin-3-O-α-l-rhamnose-(1→2)-α-l-rhamnose-7-O-β-d-glucoside | A | Rha-Rha | Glu | OH | OH | H | - | H. rhamnoides L. subsp. sinensis, H. tibetana S. | [25] |
| 18 | Quercetin(3-O-[(6-O-E-sinapoyl)-β-d-glucopyranosyl-(1→2)]-β-d-glucopyranosyl-7-O-α-l-rhamnopyranoside) | A | Glc-(2′′-O-Glc)-(6′′′-O-E-sinapoyl) | Glu | OH | OH | H | - | H. rhamnoides ssp. sinensis | [33] |
| 19 | 3’-Methoxy-Quercetin-3-O-β-d-Glucoside | A | Glu | H | OMe | OH | H | - | H. tibetana S. | [34] |
| 20 | 3’-methoxy-quercetin-3-O-α-l-rhamnose-(1→2)-β-d-glucoside | A | Rha-Glu | H | OMe | OH | H | - | H. tibetana S. | [34] |
| 21 | Isorhamnetin | A | H | H | OMe | OH | H | - | H. rhamnoides L. subsp. Wolongensis, H. rhamnoides L. subsp. sinensis, H. tibetana S. | [14,25] |
| 22 | Isorhamnetin-3-sophorose-7-rhamnetin | A | Sop | Glu | H | H | OMe | - | H. rhamnoides L. subsp. sinensis | [28] |
| 23 | Isorhamnetin-7-O-α-l-rhamnoside | A | H | Rha | H | OH | OMe | - | H. rhamnoides L. | [26] |
| 24 | Isorhamnetin-3-O-rutinoside | A | Rut | H | H | OH | OMe | - | H. rhamnoides L. subsp. sinensis, H. tibetana S. | [25,29,32] |
| 25 | Isorhamnetin-3-O-β-d-rutinoside-7-O-α-l-rhamnoside | A | Rut | Rha | H | OMe | OH | - | H. rhamnoides L. subsp. sinensis, H. tibetana S. | [25] |
| 26 | Isorhamnetin-3-O-β-d-glucoside | A | Glu | H | H | OMe | H | - | H. rhamnoides L. subsp. sinensis, H. tibetana S. | [25] |
| 27 | Isorhamnetin-3-O-β-d-glucoside-7-O-α-L-rhamnoside | A | Glu | Rha | H | OH | OMe | - | H. rhamnoides L. subsp. sinensis, H. tibetana S. | [25] |
| 28 | Isorhamnetin-3-O-α-l-rhamnoside | A | Rha | H | H | OH | OMe | - | H. rhamnoides L. | [26] |
| 29 | Isorhamnetin-3-O-β-d-sophoroside-7-O-α-l-rhamnoside | A | Sop | Rha | H | OH | OMe | - | H. rhamnoides L. subsp. sinensis, H. tibetana S. | [29] |
| 30 | Isorhamnetin-3-O-β-d-glucose-(1→6) -β-d-glucoside | A | Glu- Glu | OH | OMe | OH | H | - | H. rhamnoides L. | [26] |
| 31 | Isorhamnetin-3-O-β-d-glucose-(1→2) -β-d- rhamnoside | A | Glu- Glu | Rha | OMe | OH | H | - | H. rhamnoides L. subsp. sinensis | [29] |
| 32 | Isorhamnetin-3-O-α-l-arabinose-7-O-α-L-rhamnoside | A | Ara | Rha | OMe | OH | H | - | H. rhamnoides L. subsp. sinensis, H. rhamnoides | [25] |
| 33 | Isorhamnetin-3-O-β-d-galactose-7-O-α-L-rhamnoside | A | Gal | Rha | OMe | OH | H | - | H. rhamnoides L. subsp. sinensis, H. rhamnoides L. | [35] |
| 34 | Isorhamnetin-3-O-β-d-sophorose-O-glucuronic acid-7-O-α-l-rhamnoside | A | Sop-O-GA | Rha | OMe | OH | H | - | H. tibetana S., H. rhamnoides L. | [25] |
| 35 | Isorhamnetin-3-O-α-l-rhamnose-(1→6)-β-d-galactoside | A | Rha-Gal | H | OMe | OH | H | - | H. rhamnoides L. | [26] |
| 36 | Isorhamnetin-3-O-α-l-arabinose-(1→4) -β-d-glucoside | A | Ara- Glu | H | OMe | OH | H | - | H. rhamnoides L. | [26] |
| 37 | Isorhamnetin-3-O-β-d–(6-O-trans-sinnapoyl) sophoroside-7-O-α-l-rhamnoside | A | (6-O-trans-sinnapoyl) Sop | Rha | OMe | OH | H | - | H. rhamnoides L. subsp. sinensis | [31] |
| 38 | Isorhamnetin(3-O-[(6-O-E-sinapoyl)-β-d-glucopyranosyl-(1→2)]-b-d-glucopyranosyl-7-O-a-L-rhamnopyranoside) | A | Glc-(2′′-O-Glc)-(6′′′-O-E-sinapoyl) | Glu | OMe | OH | H | - | H. rhamnoides ssp. sinensis | [33] |
| 39 | syringetin-3-O-rutinoside | A | Rut | H | OMe | OH | OMe | - | H. rhamnoides L. subsp. sinensis | [29] |
| 40 | Kaempferol | A | H | H | H | OH | H | - | H. rhamnoides L. subsp. Wolongensis | [14] |
| 41 | Kaempferol-7-O-α-l-rhamnoside | A | H | Rha | H | OH | H | - | H. rhamnoides L. subsp. sinensis, H. tibetana S. | [29] |
| 42 | Kaempferol-3-O-glucoside | A | Glu | H | H | OH | H | - | H. rhamnoides L. subsp. sinensis, H. tibetana S. | [25] |
| 43 | Kaempferol-3-O-β-d-glucose-(1→2)-β-d-rhamnoside | A | Glu-Glu | Rha | OMe | OH | H | - | H. rhamnoides L. subsp. sinensis, | [29] |
| 44 | Kaempferol-3-O-β-d-sophorose-7-O-α-l-rhamnoside | A | Sop | Rha | H | OH | H | - | H. rhamnoides L. subsp. sinensis, H. tibetana S., H. rhamnoides L. | [25,28] |
| 45 | Kaempferol-3-O-β-d-glucose-(1→2)-α-L-rhamnose-(1→2)-α-L-rhamnoside | A | Glu-Rha-Rha | H | H | OH | H | - | H. rhamnoides L. | [26] |
| 46 | Kaempferol-3-O-β-d-rutin-7-O-α-L-rhamnoside | A | Rut | Rha | H | OH | H | - | H. rhamnoides L. subsp. sinensis, H. tibetana S., H. rhamnoides L. | [25,26] |
| 47 | Kaempferol-3-O-β-d-glucose-(1→2)-α-L-rhamnoside | A | GluRha | H | H | OH | H | - | H. rhamnoides L. subsp. sinensis, H. tibetana S., H. rhamnoides L. | [25,26] |
| 48 | Kaempferol-3-O-β-d-sophorose-O-glucuronic acid-7-O-α-L-rhamnoside | A | Sop-O-GA | Rha | H | OH | H | - | H. tibetana S., H. rhamnoides L. | [25,26] |
| 49 | Kaempferol-3-O-β-d-sophoroside-7-O-α-L-rhamnoside | A | Sop | Rha | H | OH | H | - | H. rhamnoides L. subsp. sinensis | [29] |
| 50 | Kaempferol-3-O-β-d–(6-O-trans-sinnapoyl) sophoroside-7-O-α-L-rhamnoside | A | (6-O-trans-sinnapoyl) Sop | Rha | H | OH | H | - | H. rhamnoides L. subsp. sinensis | [31] |
| 51 | Kaempferol(3-O-[(6-O-E-sinapoyl)-β-d-glucopyranosyl-(1→2)]-β-d-gluco-pyranosyl-7-O-α-L-rhamnopyranoside) | A | Glc-(2′′-O-Glc)-(6′′′-O-E-sinapoyl) | Glu | H | OH | H | - | H. rhamnoides ssp. sinensis | [33] |
| 52 | Myricetin | A | H | H | OH | OH | OH | - | H. rhamnoides L. | [26] |
| 53 | Myricetin-3-O-α-l-rhamnose-(1→6)-β-d-glucoside | A | H | H | OH | OH | H | - | H. rhamnoides L. subsp. sinensis, H. rhamnoides L. | [25,26] |
| 54 | Syringetin-3-O-β-d-rutinoside | A | Rut | H | OMe | OH | OMe | - | H. rhamnoides L. subsp. sinensis, | [29] |
| 55 | Tamarixetin-3-O-glucose | A | Glu | H | OH | OH | H | - | H. rhamnoides L. subsp. mongolica, H. rhamnoides L. | [26,28] |
| 56 | Tamarixetin-3-O-β-d-glucose-7-O-α-l-rhamnoside | A | Glu | Rha | OH | OH | H | - | H. rhamnoides L. subsp. mongolica | [28] |
| 57 | (+)-Catechin | B | H | H | H | H | OH | H | H. rhamnoides L. subsp. sinensis | [23,28] |
| 58 | (+)-Catechin-(4α→2)-phloroglucinol | B | H | OH | H | phloroglucinol | OH | H | H. rhamnoides L. subsp. rhamnoides cv., H. rhamnoides L. subsp. sinensis | [28,36] |
| 59 | (-)-Epicatechin | B | OH | H | H | H | OH | H | H. tibetana S. | [34] |
| 60 | (-)-Epicatechin-(4β→2)-phloroglucinol | B | OH | H | phloroglucinol | H | OH | H | H. rhamnoides L. subsp. sinensis | [28] |
| 61 | (-)-Epigallocatechin | B | H | OH | H | H | OH | OH | H. rhamnoides L. subsp. rhamnoides cv., H. rhamnoides L. subsp. sinensis | [23,36] |
| 62 | (+)-Gallocatechin | B | OH | H | H | H | OH | OH | H. rhamnoides L. subsp. rhamnoides cv., H. rhamnoides L. subsp. sinensis | [23,36] |
| 63 | (+)-Gallocatechin-(4α→2)-phloroglucinol | B | H | OH | H | phloroglucinol | OH | OH | H. rhamnoides L. subsp. rhamnoides cv., H. rhamnoides L. subsp. sinensis | [28,36] |
| 64 | (-)-Epicatechin gallate | B | H | 3,4,5-trihydroxybenzoic | H | H | H | OH | H. rhamnoides L. subsp. sinensis | [26] |
| 65 | (-)-Gallocatechin gallate | B | H | 3,4,5-trihydroxybenzoic | H | H | OH | OH | H. rhamnoides L. subsp. sinensis | [26] |
| 66 | Naringenin | C | H | - | - | - | - | - | H. rhamnoides L. subsp. sinensis | [24] |
| 67 | Naringin | C | Rha-Glc | - | - | - | - | - | H. rhamnoides L. subsp. sinensis | [24] |
| 68 | 2′-O-Methylisoliquiritigenin | - | - | - | - | - | - | - | H. rhamnoides L. | [37] |
| 69 | Apigenin | - | - | - | - | - | - | - | H. rhamnoides L. | [23] |
| No. | Compounds | R1 | R2 | R3 | R4 | R5 | Species | Ref. |
|---|---|---|---|---|---|---|---|---|
| 70 | Gallic acid | COOH | H | OH | OH | OH | H. rhamnoides ssp. turkestanica | [38] |
| 71 | Syringic acid | COOH | H | OMe | OH | OMe | H. rhamnoides L. subsp. sinensis | [39] |
| 72 | Protocatechuic acid | COOH | H | OH | OH | H | H. rhamnoides ssp. turkestanica | [38] |
| 73 | Salicylic acid | COOH | OH | H | H | H | H. rhamnoides ssp. turkestanica | [38] |
| 74 | Vanillic acid | COOH | H | OMe | OH | H | H. rhamnoides ssp. turkestanica | [38] |
| 75 | Gentisic acid | COOH | OH | H | OH | OH | H. rhamnoides L. subsp. sinensis | [39] |
| 76 | Caffeic acid | CHCHCOOH | H | OH | OH | H | H. rhamnoides ssp. turkestanica | [38] |
| 77 | Sinapic acid | CHCHCOOH | H | OMe | OH | OMe | H. rhamnoides L. subsp. sinensis | [39] |
| 78 | Ferulic acid | CHCHCOOH | H | OMe | OH | H | H. rhamnoides ssp. turkestanica | [38] |
| 79 | Cinnamic acid | CHCHCOOH | H | H | H | H | H. rhamnoides ssp. turkestanica | [38] |
| 80 | 1-feruloyl-β-d-glucopyranoside | CHCHCOOH-Glu | H | H | H | OMe | H. rhamnoides L. | [32] |
| 81 | P-hydroxy benzoic acid | COOH | H | H | OH | H | H. rhamnoides ssp. turkestanica | [38] |
| 82 | P-coumaric acid | CHCHCOOH | H | H | OH | H | H. rhamnoides ssp. turkestanica | [38] |
| 83 | Butylparaben | COO(CH2)3CH3 | H | H | H | H | H. tibetana S. | [34] |
| 84 | Chlorogenic acid | G | H | OH | OH | H | H. rhamnoides L. subsp. sinensis | [39] |
| No. | Compounds | R1 | R2 | R3 | R4 | Species | Ref. |
|---|---|---|---|---|---|---|---|
| 85 | Casuaricitin | O-(3,4,5-trihydroxybenzoic) | H | HHDP | H. rhamnoides L. | [41,42] | |
| 86 | Pedunculagin | H | OH | HHDP | H. rhamnoides L. | [41,42] | |
| 87 | Strictinin | O-(3,4,5-trihydroxybenzoic) | H | H | H | H. rhamnoides L. | [41,42] |
| 88 | Tellimagrandi Ⅰ | H | OH | 3,4,5-trihydroxybenzoic | 3,4,5-trihydroxybenzoic | H. rhamnoides L. | [41,42] |
| No. | Compounds | Species | Ref. |
|---|---|---|---|
| 89 | Hippophaenin A | H. rhamnoides L. | [42] |
| 90 | Hippophaenin B | H. rhamnoides L. | [42] |
| 91 | Casuarinin | H. rhamnoides L. | [42,43] |
| 92 | Stachyurin | H. rhamnoides L. | [42,43] |
| 93 | Vescalagin | H. rhamnoides L. | [42,43] |
| 94 | Castalagin | H. rhamnoides L. | [42,43] |
| 95 | Isostrictinin | H. rhamnoides L. | [41,42] |
| 96 | Hyporhamnin | H. rhamnoides L. | [42,43] |
| 97 | 1,2,6-trigalloylglucose | H. rhamnoides L. | [41,42] |
| 98 | Gossypol | H. rhamnoides L. | [43] |
| 99 | Ellagic acid | H. rhamnoides L. subsp. sinensis, H. tibetana S., H. rhamnoides L. | [25,43] |
| Ingredients | Model | Treatment | Results | Ref. |
|---|---|---|---|---|
| Apigenin | Sprague–Dawley rats | 10, 15 and 20 mg/kg | Apigenin significantly reduced the lipid hydroperoxides and increased the total antioxidant levels. | [84] |
| Quercetin | Streptozotocin (STZ)-induced diabetes in rats | 15 mg/kg/d | Quercetin plays a protective role by reducing lipid peroxidation, NO production and increasing antioxidant enzyme activity. | [85] |
| Isorhamnetin | Carrageenan-induced paw edema in Sprague–Dawley rats | 10 or 30 mg/kg | The induction of Ho-1 by isorhamin can reduce the production of ROS and significantly increase the nuclear level of Nrf2. | [86] |
| Kaempferol | Sprague–Dawley rats | 15 mmol/L | Kaempferol significantly increased SOD activity and GSH/GSSG ratio, while significantly reduced MDA level. | [87] |
| Myricetin | Human MCF-7 breast cancer cells | 0.0, 0.05, 0.1 and 0.2 µmol | Myricetin decreased ferric ions and cellular ROS, respectively. | [88] |
| Naringenin | Ethanol-induced rats | 50 mg/kg | Naringenin elevated the activities of SOD and catalase in the tissues of ethanol-treated rats, inhibit malondialdehyde and to scavenge hydroxyl groups, increased the activities of GR, GPx and GST. | [89] |
| Naringin | Isoproterenol (ISO)-induced myocardial infarction (MI) in rats | 10, 20, 40 mg/kg | Naringin saw a significant decrease in the levels of lipid peroxidative products and improved the antioxidant status by increasing the activities of antioxidant enzymes and nonenzymatic antioxidants. | [90] |
| Hyperoside | Hydrogen peroxide-induced V79-4 cells | 1, 2.5 and 5 µmol | Hyperoside was shown to possess cytoprotective properties against oxidative stress by scavenging intracellular ROS and enhancing the catalase and glutathione peroxidase activities. | [91] |
| (+)-Catechin | Mature male Wistar rats were given chlorpyrifos | 20 mg/kg | Catechin statistically significantly decreased MDA levels, SOD and CAT activities, while increased GPx and GST activities. | [92] |
| Rutin | Streptozotocin (STZ)-induced diabetic rats | 100 mg/kg | Rutin improved the antioxidant status of diabetic rats by decreasing lipid peroxidative products and increasing enzymic and nonenzymic antioxidants. | [93] |
| Gallic acid | Streptozotocin-induced diabetic male Wistar rats | 10 and 20 mg/kg | Gallic acid significant increasing lipid hydroperoxides, SOD, CAT, GPx activities. | [94] |
| Syringic acid | Nephrotoxicity was induced in male Wistar albino rats by the administration | 50 mg/kg | Syringic acid increased GPx, CAT and SOD activities of renal tissue. | [95] |
| Vanillic acid | Oral squamous cell carcinomas were induced in each hamster’s buccal pouch (left side only) | 200 mg/kg | Vanillic acid significantly restored the SOD, CAT, GPx, GSH, vitamin E, vitamin C to near normal range in DMBA-treated hamsters. | [96] |
| Chlorogenic acid | Intestinal mitochondrial injury by H2O2 | 0, 20, 40, 80, and 160 μmol/L | Chlorogenic acid decreased H2O2-induced ROS production in a dose-dependent manner, and T-AOC, SOD and GSH activities were also increased. | [97] |
| Ferulic acid | Streptozotocin-induced diabetic rats | 10 and 20 mg/kg | Ferulic acid effectively inhibited the lipid peroxidation and elevated the catalase, superoxide dismutase, glutathione and nitric oxide levels. | [98] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, M.; Gong, X.; Li, X.; Wang, C.; Li, M. Advanced Research on the Antioxidant Activity and Mechanism of Polyphenols from Hippophae Species—A Review. Molecules 2020, 25, 917. https://doi.org/10.3390/molecules25040917
Ji M, Gong X, Li X, Wang C, Li M. Advanced Research on the Antioxidant Activity and Mechanism of Polyphenols from Hippophae Species—A Review. Molecules. 2020; 25(4):917. https://doi.org/10.3390/molecules25040917
Chicago/Turabian StyleJi, Mingyue, Xue Gong, Xue Li, Congcong Wang, and Minhui Li. 2020. "Advanced Research on the Antioxidant Activity and Mechanism of Polyphenols from Hippophae Species—A Review" Molecules 25, no. 4: 917. https://doi.org/10.3390/molecules25040917
APA StyleJi, M., Gong, X., Li, X., Wang, C., & Li, M. (2020). Advanced Research on the Antioxidant Activity and Mechanism of Polyphenols from Hippophae Species—A Review. Molecules, 25(4), 917. https://doi.org/10.3390/molecules25040917