Characterization of Metabolites in Plasma, Urine and Feces of Healthy Participants after Taking Brahmi Essence for Twelve Weeks Using LC-ESI-QTOF-MS Metabolomic Approach
Abstract
:1. Introduction
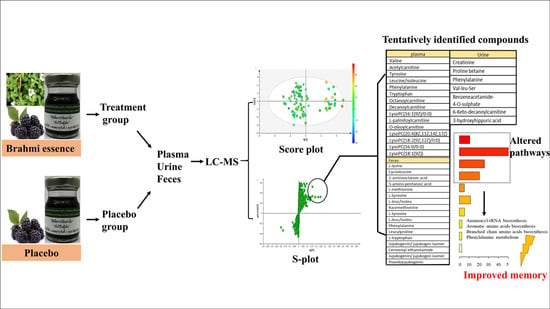
2. Results and Discussion
2.1. Analysis of Metabolites in Plasma Samples from Healthy Participants after Taking Brahmi Essence for 12 Weeks
2.2. Analysis of Metabolites in Urine Samples from Healthy Participants after Taking Brahmi Essence for 12 Weeks
2.3. Analysis of Metabolites in Feces Samples from Healthy Participants after Taking Brahmi Essence for 12 Weeks
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Brahmi Essence and Mulberry Preparation
3.3. Human Ethical Statement
3.4. Design of the Study
3.5. Plasma, Urine, and Feces Sample Collection
3.6. Assessment of Memory Speed
3.7. Sample Preparations
3.7.1. Preparation of Brahmi Essence and Placebo for LC-MS Analysis
3.7.2. Preparation of Plasma, Urine, and Feces Samples for LC-MS Analysis
3.8. LC-ESI-QTOF-MS Analysis Conditions
3.9. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Spencer, C.H.B.; Strother, J.L. Taxonomy and natural history of Bacopa (Scrophulariaceae) in California. Syst. Bot. 1978, 3, 408–419. [Google Scholar]
- Russo, A.; Borrelli, F. Bacopa monniera, a reputed nootropic plant: An overview. Phytomedicine 2005, 12, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Uabundit, N.; Wattanathorn, J.; Mucimapura, S.; Ingkaninan, K. Cognitive enhancement and neuroprotective effects of Bacopa monnieri in Alzheimer’s disease model. J. Ethnopharmacol. 2010, 127, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Saesong, T.; Allard, P.M.; Queiroz, E.F.; Marcourt, L.; Nuengchamnong, N.; Temkitthawon, P.; Khorana, N.; Wolfender, J.L.; Ingkaninan, K. Discovery of lipid peroxidation inhibitors from Bacopa species prioritized through multivariate data analysis and multi-informative molecular networking. Molecules 2019, 24, 2989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulose, C.S.; Chathu, F.; Reas Khan, S.; Krishnakumar, A. Neuroprotective role of Bacopa monnieri extract in epilepsy and effect of glucose supplementation during hypoxia: Glutamate receptor gene expression. Neurochem. Res. 2008, 33, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Nemetchek, M.D.; Stierle, A.A.; Stierle, D.B.; Lurie, D.I. The Ayurvedic plant Bacopa monnieri inhibits inflammatory pathways in the brain. J. Ethnopharmacol. 2017, 197, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Tembhre, M.; Ahirwar, S.; Gour, S.; Namdeo, A. Inhibitory potential of acephate and ethanol extract of Bacopa monnieri on AChE in rat cortex and hippocampus. Int. J. Biosci. Biochem. Bioinform. 2015, 5, 45. [Google Scholar] [CrossRef] [Green Version]
- Limpeanchob, N.; Jaipan, S.; Rattanakaruna, S.; Phrompittayarat, W.; Ingkaninan, K. Neuroprotective effect of Bacopa monnieri on beta-amyloid-induced cell death in primary cortical culture. J. Ethnopharmacol. 2008, 120, 112–117. [Google Scholar] [CrossRef]
- Kamkaew, N.; Norman Scholfield, C.; Ingkaninan, K.; Taepavarapruk, N.; Chootip, K. Bacopa monnieri increases cerebral blood flow in rat independent of blood pressure. Phytother. Res. 2013, 27, 135–138. [Google Scholar] [CrossRef]
- Kamkaew, N.; Paracha, T.; Ingkaninan, K.; Waranuch, N.; Chootip, K. Vasodilatory effects and mechanisms of action of Bacopa monnieri active compounds on rat mesenteric arteries. Molecules 2019, 24, 2243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamkaew, N.; Norman Scholfield, C.; Ingkaninan, K.; Maneesai, P.; Parkington, H.C.; Tare, M.; Chootip, K. Bacopa monnieri and its constituents is hypotensive in anaesthetized rats and vasodilator in various artery types. J. Ethnopharmacol. 2011, 137, 790–795. [Google Scholar] [CrossRef]
- Allan, J.J.; Damodaran, A.; Deshmukh, N.; Goudar, K.; Amit, A. Safety evaluation of a standardized phytochemical composition extracted from Bacopa monnieri in Sprague-Dawley rats. Food Chem. Toxicol. 2007, 45, 1928–1937. [Google Scholar] [CrossRef]
- Sireeratawong, S.; Jaijoy, K.; Khonsung, P.; Lertprasertsuk, N.; Ingkaninan, K. Acute and chronic toxicities of Bacopa monnieri extract in Sprague-Dawley rats. BMC Complement. Altern. Med. 2016, 16, 249. [Google Scholar] [CrossRef] [Green Version]
- Peth-Nui, T.; Wattanathorn, J.; Muchimapura, S.; Tong-Un, T.; Piyavhatkul, N.; Rangseekajee, P.; Ingkaninan, K.; Vittaya-areekul, S. Effects of 12-week Bacopa monnieri consumption on attention, cognitive processing, working memory, and functions of both cholinergic and monoaminergic systems in healthy elderly volunteers. Evid. Based Complement. Altern. Med. 2012, 2012, 606424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuengchamnong, N.; Sookying, S.; Ingkaninan, K. LC-ESI-QTOF-MS based screening and identification of isomeric jujubogenin and pseudojujubogenin aglycones in Bacopa monnieri extract. J. Pharm. Biomed. Anal. 2016, 129, 121–134. [Google Scholar] [CrossRef]
- Garai, S.; Mahato, S.B.; Ohtani, K.; Yamasaki, K. Dammarane-type triterpenoid saponins from Bacopa monniera. Phytochemistry 1996, 42, 815–820. [Google Scholar] [CrossRef]
- Saesong, T.; Nangngam, P.; Ingkaninan, K. Pharmacognostic and physico-chemical investigations of the aerial part of Bacopa monnieri (L.) Wettst. Songklanakarin J. Sci. Technol. 2019, 41, 397–404. [Google Scholar]
- Zhou, Y.; Shen, Y.H.; Zhang, C.; Zhang, W.D. Chemical constituents of Bacopa monnieri. Chem. Nat. Compd. 2007, 43, 355–357. [Google Scholar] [CrossRef]
- Rastogi, M.; Ojha, R.P.; Prabu, P.C.; Devi, B.P.; Agrawal, A.; Dubey, G.P. Prevention of age-associated neurodegeneration and promotion of healthy brain ageing in female Wistar rats by long term use of bacosides. Biogerontology 2012, 13, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, V.C.; Viswanathan, G.; Baby, S. Insights into the molecular aspects of neuroprotective Bacoside A and Bacopaside I. Curr. Neuropharmacol. 2019, 17, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Kamkaew, N. Effects of Bacopa Monnieri on Memory, Blood flow, and Vasorelaxation. Ph.D. Thesis, Naresuan University, Phitsanulok, Thailand, 2020. [Google Scholar]
- Chen, C.; Gonzalez, F.J.; Idle, J.R. LC-MS-based metabolomics in drug metabolism. Drug Metab. Rev. 2007, 39, 581–597. [Google Scholar] [CrossRef] [Green Version]
- Veselkov, K.A.; Vingara, L.K.; Masson, P.; Robinette, S.L.; Want, E.; Li, J.V.; Barton, R.H.; Boursier-Neyret, C.; Walther, B.; Ebbels, T.M.; et al. Optimized preprocessing of ultra-performance liquid chromatography/mass spectrometry urinary metabolic profiles for improved information recovery. Anal. Chem. 2011, 83, 5864–5872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sun, H.; Wang, Z.; Sun, W.; Wang, P.; Wang, X. Metabolomics: Towards understanding traditional Chinese medicine. Planta Med. 2010, 76, 2026–2035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, L.L.; McDonald, D.A.; Borum, P.R. Acylcarnitines: Role in brain. Prog. Lipid Res. 2010, 49, 61–75. [Google Scholar] [CrossRef]
- Cristofano, A.; Sapere, N.; La Marca, G.; Angiolillo, A.; Vitale, M.; Corbi, G.; Scapagnini, G.; Intrieri, M.; Russo, C.; Corso, G. Serum levels of acyl-carnitines along the continuum from normal to Alzheimer’s dementia. PLoS ONE 2016, 11, e0155694. [Google Scholar] [CrossRef]
- Lagarde, M.; Bernoud, N.; Brossard, N.; Lemaitre-Delaunay, D.; Thiès, F.; Croset, M.; Lecerf, J. Lysophosphatidylcholine as a preferred carrier form of docosahexaenoic acid to the brain. J. Mol. Neurosci. 2001, 16, 201–204. [Google Scholar] [CrossRef]
- Grimm, M.O.W.; Kuchenbecker, J.; Grösgen, S.; Burg, V.K.; Hundsdörfer, B.; Rothhaar, T.L.; Friess, P.; de Wilde, M.C.; Broersen, L.M.; Penke, B.; et al. Docosahexaenoic acid reduces amyloid β production via multiple pleiotropic mechanisms. J. Biol. Chem. 2011, 286, 14028–14039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raina, M.; Ibba, M. tRNAs as regulators of biological processes. Front. Genet. 2014, 5, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimomura, Y.; Kitaura, Y. Physiological and pathological roles of branched-chain amino acids in the regulation of protein and energy metabolism and neurological functions. Pharmacol. Res. 2018, 133, 215–217. [Google Scholar] [CrossRef]
- Fernstrom, J.D.; Fernstrom, M.H. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J. Nutr. 2007, 137, 1539S–1547S. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Lang, T.; Bader, M.; Beusch, A.; Schlagbauer, V.; Hofmann, T. High-Throughput Quantitation of Proline Betaine in Foods and Suitability as a Valid Biomarker for Citrus Consumption. J. Agric. Food Chem. 2017, 65, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Rechner, A.R.; Kuhnle, G.; Hu, H.; Roedig-Penman, A.; van den Braak, M.H.; Moore, K.P.; Rice-Evans, C.A. The metabolism of dietary polyphenols and the relevance to circulating levels of conjugated metabolites. Free Radic. Res. 2002, 36, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Sookying, S.; Pekthong, D.; Oo-puthinan, S.; Nuengchamnong, N.; Ingkaninan, K. Quantitation of bacopaside I in rat biological samples by LC-QTOF-MS/MS and its pharmacokinetic application. Nat. Prod. Commun. 2017, 12, 893–896. [Google Scholar] [CrossRef] [Green Version]
- Phrompittayarat, W.; Wittaya-areekul, S.; Jetiyanon, K.; Putalun, W.; Tanaka, H.; Ingkaninan, K. Determination of saponin glycosides in Bacopa monnieri by reversed phase High Performance Liquid Chromatography. Thai Pharm. Health Sci. J. Thai J. Pharm. Sci. 2007, 2, 26–32. [Google Scholar]






| Metabolite ID | Rt (min) | p[1], p(corr)[1] Value | Detected m/z | Assigned Ion | Detected MS/MS (% Abundance) | Error (ppm) | Tentative Identification * |
|---|---|---|---|---|---|---|---|
| I. | Metabolites in plasma | ||||||
| 1 | 3.0 | 0.06, 0.20 | 101.0030 | - | 56.9650 (100) | - | UI |
| 2 | 3.1 | 0.07, 0.30 | 118.0863 | [M + H] + | 72.0807 (100),55.0543 (79),61.0399 (19),102.0112 (1) | −0.4 | Valine A, E, F |
| 3 | 3.2 | 0.06, 0.30 | 204.1241 | [M + H] + | 85.0289 (100), 60.0808 (12), 57.0334 (4), 144.1024 (2) | −6.7 | Acetylcarnitine A, E, F |
| 4 | 3.5 | 0.06, 0.30 | 182.0810 | [M + H] + | 91.0546 (100), 123.0444 (60), 77.0386 (13), 51.0231 (7), 65.0388 (3), 136.0336 (1) | 0.9 | Tyrosine A, E, F |
| 5 | 3.9 | 0.22, 0.30 | 132.1019 | [M + H] + | 86.0966 (100) | 0.8 | Leucine/isoleucine A, E, F |
| 6 | 5.3 | 0.15, 0.30 | 166.0861 | [M + H] + | 120.0814 (100), 103.0547 (43), 77.0388 (16), 131.0495 (1) | 1.5 | Phenylalanine A, E, F |
| 7 | 6.7 | 0.13, 0.30 | 205.0972 | [M + H] + | 146.061 (100), 118.0659 (87), 188.0718 (54), 84.9601 (24), 132.0812 (20), 91.0547 (13), 159.0924 (12), 65.0392 (1) | 0.3 | Tryptophan A, E, F |
| 8 | 13.1 | 0.06, 0.50 | 288.2169 | [M + H] + | 85.0293 (100), 60.0815 (9), 229.1462 (6), 57.0707 (4), 144.1014 (2) | 0.1 | Octanoylcarnitine A, E, F |
| 9 | 15.7 | 0.06, 0.50 | 316.2480 | [M + H] + | 85.0293 (100), 60.0813 (10), 257.1778 (7), 144.1028 (2), 57.0338 (2), 95.0855 (2), 214.9232 (1) | 0.7 | Decanoylcarnitine A, E, F |
| 10 | 16.5 | 0.12, 0.30 | 332.0700 | - | 95.0614 (100), 121.0409 (46), 164.0834 (22), 96.0645 (12) | - | UI |
| 11 | 18.8 | 0.08, 0.40 | 219.1872 | - | 78.0236 (13.5), 163.1257 (7), 191.1575 (1) | - | UI |
| 12 | 22.6 | 0.07, 0.30 | 494.3238 | [M + H] + | 184.0749 (44), 104.1075 (42), 311.261 (1), 258.1111 (1) | 0.8 | LysoPC (16:1(9Z)/0:0) A, D |
| 13 | 22.7 | 0.05, 0.40 | 400.3415 | [M + H] + | 85.0292 (93), 341.272 (11), 144.1042 (4), 239.2392 (3) | 1.6 | Palmitoylcarnitine A, E, F |
| 14 | 23.3 | 0.05, 0.30 | 426.3572 | [M + H] + | 85.0295 (50), 60.0809 (6), 367.2878 (4), 144.1027 (1), 297.2103 (1), 129.0791 (1), 95.0495 (1) | 1.4 | O-oleoylcarnitine A, D, E |
| 15 | 23.7 | 0.07, 0.30 | 544.3398 | [M + H] + | 184.0748 (31), 104.1079 (30), 526.3322 (5), 86.0968 (2), 60.0814 (2), 485.2559 (1) | −0.1 | LysoPC (20:4 (8Z,11Z, 14Z, 17Z)) A, C, D |
| 16 | 23.8 | 0.13, 0.30 | 520.3392 | [M + H] + | 104.1078 (34), 184.075 (33), 258.1124 (1), 337.2781 (1), 443.2566 (1) | 1.1 | LysoPC (18:2(9Z,12Z)/0:0) A, C, D |
| 17 | 25.5 | 0.24, 0.30 | 496.3398 | [M + H] + | 184.0752 (100), 104.1076 (1) | −0.1 | LysoPC (16:0/0:0) A, C, D |
| 18 | 26.6 | 0.13, 0.30 | 522.3556 | [M + H] + | 104.1077 (33), 184.075 (30) | 0.7 | LysoPC (18:1(9Z)) A, D |
| II | Metabolites in urine | ||||||
| 19 | 3.1 | 0.18, 0.10 | 114.0675 | [M + H] + | 59.0505 (48), 86.0733 (10), 72.0458 (1) | −12.5 | Creatinine A, D, F |
| 20 | 3.4 | 0.12, 0.20 | 144.1027 | [M + H] + | 58.0664 (100), 84.0827 (26), 72.0826 (14) | −5.5 | Proline betaine A, D |
| 21 | 7.5 | 0.06, 0.20 | 166.0870 | [M + H] + | 120.0839 (100), 103.0566 (5), 131.0521 (4), 77.0406 (1) | −4.5 | Phenylalanine A, E, F |
| 22 | 8.8 | 0.12, 0.30 | 318.2025 | [M + H] + | 157.0898 (31), 85.0302 (13), 241.1129 (13) 60.0820 (9), 111.0832 (2), 274.1261 (1) | −0.5 | Valine-leucine-Serine B |
| 23 | 9.1 | 0.06, 0.20 | 256.1915 | [M + H2O] + | 85.0304 (86), 197.0503 (22), 57.0343 (5), 144.106 (4), 60.0821 (3) | −3.0 | Heptanoylcarnitine A |
| 24 | 9.5 | 0.05, 0.19 | 232.0283 | [M + H] + | 85.0306 (100), 173.0852 (25), 60.0821 (12) | −3.8 | Benzeneacetamide-4-O-sulphate A |
| 25 | 13.6 | 0.08, 0.20 | 196.0611 | [M + H] + | 121.032 (100), 65.0401 (2), 93.0757 (1) | −3.4 | 3-hydroxyhippuric acid A, D, E |
| 26 | 13.6 | 0.05, 0.20 | 432.2406 | - | 253.1501 (6), 85.0303 (2), 315.1423 (2), 297.1328 (1), 60.0813 (1), 144.1048 (1) | - | UI |
| 27 | 13.9 | 0.11, 0.20 | 300.2178 | - | 85.0302 (96), 251.1331 (13), 121.1035 (13) | - | UI |
| 28 | 14.7 | 0.05, 0.20 | 325.2272 | - | 86.098 (77), 91.0565 (51), 233.1712 (51), 85.0309 (9) | - | UI |
| III | Metabolites in feces | ||||||
| 29 | 2.8 | 0.08, 0.50 | 147.1143 | [M + H] + | 84.0464 (100), 102.0572 (33), 130.0529 (33), 56.0507 (6), 85.0493 (5) | −4.0 | Lysine A, F |
| 30 | 3.7 | 0.12, 0.30 | 130.0875 | [M + H] + | 84.083 (100), 59.0741 (1) | −9.6 | Cycloleucine A, F |
| 31 | 3.7 | 0.12, 0.30 | 160.1347 | [M + H] + | 101.062 (26), 60.0822 (2), 55.0553 (11), 83.0509 (7) | −9.3 | 2-aminooctanoic acid A |
| 32 | 4.0 | 0.09, 0.40 | 118.0878 | [M + H] + | 72.0824 (100), 59.0504 (29), 55.0553 (18) | −13.1 | 5-amino-pentanoic acid A |
| 33 | 4.1 | 0.08, 0.50 | 150.0596 | [M + H] + | 56.0507 (100), 104.0551 (81), 133.035 (63), 61.012 (34), 87.0281 (8) | −8.5 | Methionine A, F |
| 34 | 4.2 | 0.06, 0.40 | 182.0833 | [M + H] + | 136.0756 (100), 165.0544 (59), 123.0441 (34), 147.0439 (15), 91.0541 (10) | −9.0 | Tyrosine A, E, F |
| 35 | 4.7 | 0.09, 0.40 | 132.1032 | [M + H] + | 86.0959 (100), 69.0693 (9), 59.0483 (1) | 3.8 | Leucine/isoleucine A, E, F |
| 36 | 6.2 | 0.06, 0.40 | 182.0828 | [M + H] + | 136.0788 (100), 165.0585 (55), 123.0469 (34), 119.0518 (19), 147.0473 (15), 91.0562 (11) | −9.0 | Tyrosine A, E, F |
| 37 | 5.6 | 0.05, 0.3 | 150.0596 | [M + H] + | 104.0585 (100), 56.0526 (99), 133.0393 (65), 61.0139 (46), 87.0308 (8), 74.0274 (7) | −8.5 | Racemethionine A, D |
| 38 | 6.9 | 0.32, 0.40 | 132.1002 | [M + H] + | 86.0951 (100), 69.0686 (6) | 12.8 | Leucine/isoleucine A, E, F |
| 39 | 7.0 | 0.05, 0.40 | 264.1457 | [M + H] + | 246.1352 (100), 200.1293 (62), 228.1245 (48), 131.0581 (16), 86.0968 (14) | - | UI |
| 40 | 8.0 | 0.19, 0.40 | 166.0880 | [M + H] + | 120.084 (100), 103.0567 (5), 131.0522 (3), 149.0633 (1) | −10.5 | Phenylalanine A, E, F |
| 41 | 8.5 | 0.06, 0.30 | 229.1561 | [M + H] + | 86.0977 (100), 116.0725 (97), 70.0662 (11) | −6.2 | Leucyl-proline A, D |
| 42 | 9.2 | 0.10, 0.40 | 205.0988 | [M + H] + | 188.0755 (100), 146.0635 (42), 118.0679 (7) | −8.0 | L-tryptophan A, E, F |
| 43 | 12.8 | 0.11, 0.40 | 588.4181 | - | 566.4354 (100) | - | UI |
| 44 | 15.5 | 0.05, 0.30 | 398.3456 | - | 98.0989 (1) | - | UI |
| 45 | 19.1 | 0.06, 0.30 | 348.0668 | - | 111.0582 (100), 137.0380 (46), 180.0815 (28) | - | UI |
| 46 | 20.4 | 0.05, 0.60 | 455.3547 | [M + H] + | 369.2884 (16), 109.1039 (9), 437.3512 (9), 163. 0784 (3), 135.1195 (2) | −6.0 | Jujubogenin/ jujubogen isomer [15] |
| 47 | 23.0 | 0.06, 0.30 | 373.2754 | [M + H] + | 355.2726 (100), 337.2612 (6), 245.1600 (5), 107.0880 (2) | −4.5 | Cervonoyl ethanolamide A |
| 48 | 27.7 | 0.07, 0.60 | 455.3542 | [M + H] + | 369.2884 (16), 437.3526 (10), 109.1036 (8), 123.1199 (8) | −4.9 | Jujubogenin/ jujubogen isomer [15] |
| 49 | 28.2 | 0.10, 0.60 | 473.3656 | [M + H] + | 455.3624 (10), 437.3529 (7), 109.1041 (4), 123.1198 (3), 369.2888 (3) | −6.5 | Psuedojujubogenin [15] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minale, G.; Saesong, T.; Temkitthawon, P.; Waranuch, N.; Nuengchamnong, N.; Chootip, K.; Kamkaew, N.; Kongbangkerd, T.; Engsuwan, J.; Ingkaninan, K. Characterization of Metabolites in Plasma, Urine and Feces of Healthy Participants after Taking Brahmi Essence for Twelve Weeks Using LC-ESI-QTOF-MS Metabolomic Approach. Molecules 2021, 26, 2944. https://doi.org/10.3390/molecules26102944
Minale G, Saesong T, Temkitthawon P, Waranuch N, Nuengchamnong N, Chootip K, Kamkaew N, Kongbangkerd T, Engsuwan J, Ingkaninan K. Characterization of Metabolites in Plasma, Urine and Feces of Healthy Participants after Taking Brahmi Essence for Twelve Weeks Using LC-ESI-QTOF-MS Metabolomic Approach. Molecules. 2021; 26(10):2944. https://doi.org/10.3390/molecules26102944
Chicago/Turabian StyleMinale, Genet, Tongchai Saesong, Prapapan Temkitthawon, Neti Waranuch, Nitra Nuengchamnong, Krongkarn Chootip, Natakorn Kamkaew, Teeraporn Kongbangkerd, Jinutda Engsuwan, and Kornkanok Ingkaninan. 2021. "Characterization of Metabolites in Plasma, Urine and Feces of Healthy Participants after Taking Brahmi Essence for Twelve Weeks Using LC-ESI-QTOF-MS Metabolomic Approach" Molecules 26, no. 10: 2944. https://doi.org/10.3390/molecules26102944
APA StyleMinale, G., Saesong, T., Temkitthawon, P., Waranuch, N., Nuengchamnong, N., Chootip, K., Kamkaew, N., Kongbangkerd, T., Engsuwan, J., & Ingkaninan, K. (2021). Characterization of Metabolites in Plasma, Urine and Feces of Healthy Participants after Taking Brahmi Essence for Twelve Weeks Using LC-ESI-QTOF-MS Metabolomic Approach. Molecules, 26(10), 2944. https://doi.org/10.3390/molecules26102944