Detecting and Quantifying Polyhaloaromatic Environmental Pollutants by Chemiluminescence-Based Analytical Method
Abstract
:1. Introduction
1.1. Polyhaloaromatics (XAr) and Their Toxicity
1.2. The Detection of XAr
1.3. The Degradation and Treatment of XAr
1.4. Unprecedented ●OH Generation and CL Emission Can Be Produced from H2O2 and Polyhaloquinones, the Carcinogenic Metabolites of XAr
1.5. The Goal of This Paper
2. Chemiluminescence-Based Analytical Methods Induced by Fe(II)-Fenton System for the Detection of XAr
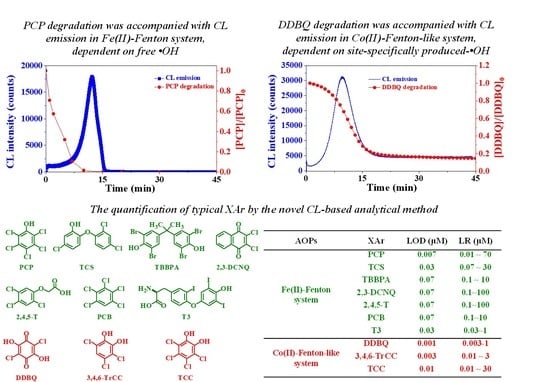
2.1. Intrinsic ●OH-Dependent CL Emission Can Be Generated from the Degradation of the Priority Pollutant PCP in Fe(II)-Fenton System
2.2. Analogous ●OH-Dependent CL Emission from the Degradation of All 19 Chlorophenols and the Underlying Structure−Activity Relationship
2.3. Similar to Chlorophenols, Other Classes of XAr Could Also Generate ●OH-Dependent Intrinsic CL Emission in the Degradation Mediated by Fe(II)-Fenton System
3. Chemiluminescence-Based Analytical Methods Induced by Co(II)-Fenton-Like System for the Detection of XAr
3.1. Distinct Intrinsic CL Emission in the Degradation of Halohydroxyl Quinoid Compounds by Co(II)-Fenton-Like System: Markedly Different from the CL Produced by Classic Fe(II)-Fenton System
3.2. Site Specifically Produced ●OH, but Not Free ●OH Is Responsible for the CL Production of Halohydroxyl Quinoid Compounds Induced by Co(II)-Fenton-Like System
3.3. The Molecular Mechanism for the Site-Specific ●OH-Dependent CL Emission of Halohydroxyl Quinoid Compounds in Co(II)-Fenton-Like System
3.4. Highly-Sensitive CL-Based Analytical Method for the Detection of Halohydroxyl Quinoid Compounds on the Basis of Co(II)-Fenton-Like System
4. The Advantages and Challenges of the Typical CL-Based Analytic Methods for the Detection of XAr in their Environmental Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olaniran, A.O.; Igbinosa, E.O. Chlorophenols and other related derivatives of environmental concern: Properties, distribution and microbial degradation processes. Chemosphere 2011, 83, 1297–1306. [Google Scholar] [CrossRef]
- Pera-Titus, M.; García-Molina, V.; Baños, M.A.; Giménez, J.; Esplugas, S. Degradation of chlorophenols by means of advanced oxidation processes: A general review. Appl. Catal. B-Environ. 2004, 47, 219–256. [Google Scholar] [CrossRef]
- Zhu, B.-Z.; Shan, G.-Q. Potential mechanism for pentachlorophenol-Induced carcinogenicity: A novel mechanism for metal-independent production of hydroxyl radicals. Chem. Res. Toxicol. 2009, 22, 969–977. [Google Scholar] [CrossRef]
- Dann, A.B.; Hontela, A. Triclosan: Environmental exposure, toxicity and mechanisms of action. J. Appl. Toxicol. 2010, 31, 285–311. [Google Scholar] [CrossRef]
- Chignell, C.F.; Han, S.K.; Mouithys-Mickalad, A.; Sik, R.H.; Stadler, K.; Kadiiska, M.B. EPR studies of in vivo radical production by 3,3′,5,5′-tetrabromobisphenol A (TBBPA) in the Sprague–Dawley rat. Toxicol. Appl. Pharmacol. 2008, 230, 17–22. [Google Scholar] [CrossRef]
- Song, Y.; Wagner, B.A.; Witmer, J.R.; Lehmler, H.-J.; Buettner, G.R. Nonenzymatic displacement of chlorine and formation of free radicals upon the reaction of glutathione with PCB quinones. Proc. Natl. Acad. Sci. USA 2009, 106, 9725–9730. [Google Scholar] [CrossRef] [Green Version]
- Michałowicz, J.; Majsterek, I. Chlorophenols, chlorocatechols and chloroguaiacols induce DNA base oxidation in human lymphocytes (in vitro). Toxicology 2010, 268, 171–175. [Google Scholar] [CrossRef]
- Carstens, C.P.; Blum, J.K.; Witte, I. The role of hydroxyl radicals in tetrachlorohydroquinone induced DNA strand break formation in PM2 DNA and human fibroblasts. Chem. Biol. Interact. 1990, 74, 305–314. [Google Scholar] [CrossRef]
- Bukowska, B. 2,4,5-T and 2,4,5-TCP induce oxidative damage in human erythrocytes: The role of glutathione. Cell Biol. Int. 2004, 28, 557–563. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Ho, Y.-S.; Jeng, J.-H.; Su, H.-J.; Lee, C.-C. Different cell death mechanisms and gene expression in human cells induced by pentachlorophenol and its major metabolite, tetrachlorohydroquinone. Chem. Biol. Interact. 2000, 128, 173–188. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Lee, C.-C.; Chang, W.-C.; Liou, H.-B.; Ho, Y.-S. Oxidative stress and liver toxicity in rats and human hepatoma cell line induced by pentachlorophenol and its major metabolite tetrachlorohydroquinone. Toxicol. Lett. 2001, 122, 157–169. [Google Scholar] [CrossRef]
- Igbinosa, E.O.; Odjadjare, E.E.; Chigor, V.N.; Igbinosa, I.H.; Emoghene, A.O.; Ekhaise, F.O.; Igiehon, N.O.; Idemudia, O.G. Toxicological profile of chlorophenols and their derivatives in the environment: The public health perspective. Sci. World J. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Guyton, K.Z.; Loomis, D.; Grosse, Y.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of pentachlorophenol and some related compounds. Lancet Oncol. 2016, 17, 1637–1638. [Google Scholar] [CrossRef]
- McConnell, E.E.; Huff, J.E.; Hejtmancik, M.; Peters, A.C.; Persing, R. Toxicology and carcinogenesis studies of two grades of pentachlorophenol in B6C3F1 mice. Fundam. Appl. Toxicol. 1991, 17, 519–532. [Google Scholar] [CrossRef]
- Seiler, J.P. Pentachlorophenol. Mutat. Res. Genet. Toxicol. 1991, 257, 27–47. [Google Scholar] [CrossRef]
- Covaci, A.; Harrad, S.; Abdallah, M.A.-E.; Ali, N.; Law, R.J.; Herzke, D.; De Wit, C.A. Novel brominated flame retardants: A review of their analysis, environmental fate and behaviour. Environ. Int. 2011, 37, 532–556. [Google Scholar] [CrossRef]
- Zimbron, J.A.; Reardon, K.F. Fenton’s oxidation of pentachlorophenol. Water Res. 2009, 43, 1831–1840. [Google Scholar] [CrossRef]
- Gupta, S.S.; Stadler, M.; Noser, C.A.; Ghosh, A.; Steinhoff, B.; Lenoir, D.; Horwitz, C.P.; Schramm, K.-W.; Collins, T. Rapid total destruction of chlorophenols by activated hydrogen peroxide. Science 2002, 296, 326–328. [Google Scholar] [CrossRef] [Green Version]
- Sorokin, A.; Seris, J.L.; Meunier, B. Efficient oxidative dechlorination and aromatic ring cleavage of chlorinated phenols catalyzed by iron sulfophthalocyanine. Science 1995, 268, 1163–1166. [Google Scholar] [CrossRef] [PubMed]
- Peller, J.; Wiest, O.; Kamat, P.V. Mechanism of hydroxyl radical-induced breakdown of the herbicide 2,4-dichlorophenoxyacetic acid (2,4-D). Chem. Eur. J. 2003, 9, 5379–5387. [Google Scholar] [CrossRef]
- Zhang, H.C.; Huang, C.H. Oxidative transformation of triclosan and chlorophene by manganese oxides. Environ. Sci. Technol. 2003, 37, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.; Rahm, S.; Green, N.; Bergman, Å.; Jakobsson, E. Photochemical transformations of tetrabromobisphenol A and related phenols in water. Chemosphere 2004, 54, 117–126. [Google Scholar] [CrossRef]
- Peiró, A.M.; Ayllón, J.A.; Peral, J.; Doménech, X. TiO2-photocatalyzed degradation of phenol and ortho-substituted phenolic compounds. Appl. Catal. B-Environ. 2001, 30, 359–373. [Google Scholar] [CrossRef]
- Schuster, G.B. Chemiluminescence of organic peroxides. Conversion of ground-state reactants to excited-state products by the chemically initiated electron-exchange luminescence mechanism. Acc. Chem. Res. 1979, 12, 366–373. [Google Scholar] [CrossRef]
- Matsumoto, M. Advanced chemistry of dioxetane-based chemiluminescent substrates originating from bioluminescence. J. Photochem. Photobiol. C 2004, 5, 27–53. [Google Scholar] [CrossRef]
- Widder, E.A. Bioluminescence in the ocean: Origins of biological, chemical, and ecological diversity. Science 2010, 328, 704–708. [Google Scholar] [CrossRef] [Green Version]
- McCapra, F. Chemical generation of excited states: The basis of chemiluminescence and bioluminescence. Methods Enzymol. 2000, 305, 3–47. [Google Scholar] [CrossRef]
- Grayeski, M.L. Chemiluminescence analysis. Anal. Chem. 1987, 59, 1243A–1256A. [Google Scholar] [CrossRef]
- Wang, X.; Lin, J.-M.; Liu, M.-L.; Cheng, X.-L. Flow-based luminescence-sensing methods for environmental water analysis. Trends Anal. Chem. 2009, 28, 75–87. [Google Scholar] [CrossRef]
- Marquette, C.A.; Blum, L.J. Chemiluminescent enzyme immunoassays: A review of bioanalytical applications. Bioanalysis 2009, 1, 1259–1269. [Google Scholar] [CrossRef]
- Dodeigne, C.; Thunus, L.; Lejeune, R. Chemiluminescence as diagnostic tool. A review. Talanta 2000, 51, 415–439. [Google Scholar] [CrossRef]
- Von Sonntag, C. Advanced oxidation processes: Mechanistic aspects. Water Sci. Technol. 2008, 58, 1015–1021. [Google Scholar] [CrossRef]
- Wang, J.-L.; Xu, L.-J. Advanced oxidation processes for wastewater treatment: Formation of hydroxyl radical and application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Rastogi, A.; Al-Abed, S.R.; Dionysiou, D.D. Effect of inorganic, synthetic and naturally occurring chelating agents on Fe(II) mediated advanced oxidation of chlorophenols. Water Res. 2009, 43, 684–694. [Google Scholar] [CrossRef]
- Lente, G.; Espenson, J.H. Photoaccelerated oxidation of chlorinated phenols. Chem. Commun. 2003, 1162–1163. [Google Scholar] [CrossRef]
- Hong, P.K.; Zeng, Y. Degradation of pentachlorophenol by ozonation and biodegradability of intermediates. Water Res. 2002, 36, 4243–4254. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015; p. 944. [Google Scholar]
- Edwards, J.O.; Curci, R. Fenton type activation and chemistry of hydroxyl radical. In Catalytic Oxidations with Hydrogen Peroxide as Oxidant; Strukul, G., Ed.; Springer: Dordrecht, The Netherlands, 1992; pp. 97–151. [Google Scholar]
- Qin, H.; Huang, C.-H.; Mao, L.; Xia, H.-Y.; Kalyanaraman, B.; Shao, J.; Shan, G.-Q.; Zhu, B.-Z. Molecular mechanism of metal-independent decomposition of lipid hydroperoxide 13-HPODE by halogenated quinoid carcinogens. Free Radic. Biol. Med. 2013, 63, 459–466. [Google Scholar] [CrossRef]
- Zhu, B.-Z.; Zhu, J.-G.; Mao, L.; Kalyanaraman, B.; Shan, G.-Q. Detoxifying carcinogenic polyhalogenated quinones by hydroxamic acids via an unusual double Lossen rearrangement mechanism. Proc. Natl. Acad. Sci. USA 2010, 107, 20686–20690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.-H.; Shan, G.-Q.; Mao, L.; Kalyanaraman, B.; Qin, H.; Ren, F.-R.; Zhu, B.-Z. The first purification and unequivocal characterization of the radical form of the carbon-centered quinone ketoxy radical adduct. Chem. Commun. 2013, 49, 6436–6438. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Ren, F.-R.; Shan, G.-Q.; Qin, H.; Mao, L.; Zhu, B.-Z. Molecular mechanism of metal-independent decomposition of organic hydroperoxides by halogenated quinoid carcinogens and the potential biological implications. Chem. Res. Toxicol. 2015, 28, 831–837. [Google Scholar] [CrossRef]
- Shao, J.; Huang, C.-H.; Kalyanaraman, B.; Zhu, B.-Z. Potent methyl oxidation of 5-methyl-2′-deoxycytidine by halogenated quinoid carcinogens and hydrogen peroxide via a metal-independent mechanism. Free Radic. Biol. Med. 2013, 60, 177–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, B.-Z.; Mao, L.; Huang, C.-H.; Qin, H.; Fan, R.-M.; Kalyanaraman, B.; Zhu, J.-G. Unprecedented hydroxyl radical-dependent two-step chemiluminescence production by polyhalogenated quinoid carcinogens and H2O2. Proc. Natl. Acad. Sci. USA 2012, 109, 16046–16051. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.-Z.; Zhao, H.-T.; Kalyanaraman, B.; Liu, J.; Shan, G.-Q.; Du, Y.-G.; Frei, B. Mechanism of metal-independent decomposition of organic hydroperoxides and formation of alkoxyl radicals by halogenated quinones. Proc. Natl. Acad. Sci. USA 2007, 104, 3698–3702. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.-Z.; Shan, G.-Q.; Huang, C.-H.; Kalyanaraman, B.; Mao, L.; Du, Y.-G. Metal-independent decomposition of hydroperoxides by halogenated quinones: Detection and identification of a quinone ketoxy radical. Proc. Natl. Acad. Sci. USA 2009, 106, 11466–11471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, B.-Z.; Zhao, H.-T.; Kalyanaraman, B.; Frei, B. Metal-independent production of hydroxyl radicals by halogenated quinones and hydrogen peroxide: An ESR spin trapping study. Free Radic. Biol. Med. 2002, 32, 465–473. [Google Scholar] [CrossRef]
- Mao, L.; Liu, Y.-X.; Huang, C.-H.; Gao, H.-Y.; Kalyanaraman, B.; Zhu, B.-Z. Intrinsic chemiluminescence generation during advanced oxidation of persistent halogenated aromatic carcinogens. Environ. Sci. Technol. 2015, 49, 7940–7947. [Google Scholar] [CrossRef]
- Gao, H.-Y.; Mao, L.; Li, F.; Xie, L.-N.; Huang, C.-H.; Shao, J.; Shao, B.; Kalyanaraman, B.; Zhu, B.-Z. Mechanism of intrinsic chemiluminescence production from the degradation of persistent chlorinated phenols by the Fenton system: A structure–activity relationship study and the critical role of quinoid and semiquinone radical intermediates. Environ. Sci. Technol. 2017, 51, 2934–2943. [Google Scholar] [CrossRef]
- Gao, H.-Y.; Mao, L.; Shao, B.; Huang, C.-H.; Zhu, B.-Z. Why does 2,3,5,6-tetrachlorophenol generate the strongest intrinsic chemiluminescence among all nineteen chlorophenolic persistent organic pollutants during environmentally-friendly advanced oxidation process? Sci. Rep. 2016, 6, 33159. [Google Scholar] [CrossRef] [Green Version]
- Mao, L.; Gao, H.-Y.; Huang, C.-H.; Qin, L.; Huang, R.; Shao, B.; Shao, J.; Zhu, B.-Z. Unprecedented strong intrinsic chemiluminescence generation from degradation of halogenated hydroxy-quinoid pollutants by Co(II)-mediated advanced oxidation processes: The critical role of site -specific production of hydroxyl radicals. Chem. Eng. J. 2020, 394. [Google Scholar] [CrossRef]
- Fang, X.W.; Schuchmann, H.-P.; Von Sonntag, C. The reaction of the ●OH radical with pentafluoro-, pentachloro-, pentabromo- and 2,4,6-triiodophenol in water: Electron transfer vs. addition to the ring. J. Chem. Soc. Perkin Trans. 2 2000, 1391–1398. [Google Scholar] [CrossRef]
- Mvula, E.; Von Sonntag, C. Ozonolysis of phenols in aqueous solution. Org. Biomol. Chem. 2003, 1, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Czaplicka, M. Photo-degradation of chlorophenols in the aqueous solution. J. Hazard. Mater. 2006, 134, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.; Meyerstein, D.; Czapski, G. The Fenton reagents. Free Radic. Biol. Med. 1993, 15, 435–445. [Google Scholar] [CrossRef]
- Tang, W.Z.; Huang, C. Effect of chlorine content of chlorinated phenols on their oxidation kinetics by Fenton’s reagent. Chemosphere 1996, 33, 1621–1635. [Google Scholar] [CrossRef]
- Tang, W.Z.; Huang, C. The effect of chlorine position of chlorinated phenols on their dechlorination kinetics by Fenton’s reagent. Waste Manag. 1995, 15, 615–622. [Google Scholar] [CrossRef]
- Oturan, N.; Panizza, M.; Oturan, M.A. Cold incineration of chlorophenols in aqueous solution by advanced electrochemical process electro-Fenton. Effect of number and position of chlorine atoms on the degradation kinetics. J. Phys. Chem. A 2009, 113, 10988–10993. [Google Scholar] [CrossRef]
- Smith, S.; Furay, V.; Layiwola, P.; Filho, J.M. Evaluation of the toxicity and quantitative structure—Activity relationships (QSAR) of chlorophenols to the copepodid stage of a marine copepod (Tisbe battagliai) and two species of benthic flatfish, the flounder (Platichthys flesus) and sole (Solea solea). Chemosphere 1994, 28, 825–836. [Google Scholar] [CrossRef]
- Padmanabhan, J.; Parthasarathi, R.; Subramanian, V.; Chattaraj, P.K. Group philicity and electrophilicity as possible descriptors for modeling ecotoxicity applied to chlorophenols. Chem. Res. Toxicol. 2006, 19, 356–364. [Google Scholar] [CrossRef]
- McKague, A.B. Some toxic constituents of chlorination-stage effluents from bleached kraft pulp mills. Can. J. Fish. Aquat. Sci. 1981, 38, 739–743. [Google Scholar] [CrossRef]
- Molčanov, K.; Jurić, M.; Kojić-Prodić, B. Stacking of metal chelating rings with π-systems in mononuclear complexes of copper(II) with 3,6-dichloro-2,5-dihydroxy-1,4-benzoquinone (chloranilic acid) and 2,2′-bipyridine ligands. Dalton Trans. 2013, 42, 15756–15765. [Google Scholar] [CrossRef]
- Verdaguer, M.; Michalowicz, A.; Girerd, J.J.; Berding, N.A.; Kahn, O. EXAFS study and magnetic properties of copper(II) chloranilato and bromanilato chains: A new example of orbital reversal. Inorg. Chem. 1980, 19, 3271–3279. [Google Scholar] [CrossRef]
- Tanaka, H.; Fukuoka, T.; Okamoto, K. Cobalt(III) oxidation pretreatment of organic phosphorus compounds for a spectrophotometric determination of phosphorus in water. Anal. Sci. 1994, 10, 769–774. [Google Scholar] [CrossRef] [Green Version]
- McIntyre, N.S.; Cook, M.G. X-ray photoelectron studies on some oxides and hydroxides of cobalt, nickel, and copper. Anal. Chem. 1975, 47, 2208–2213. [Google Scholar] [CrossRef]
- Fu, L.; Liu, Z.; Liu, Y.; Han, B.; Hu, P.; Cao, L.; Zhu, D. Beaded cobalt oxide nanoparticles along carbon nanotubes: Towards more highly integrated electronic devices. Adv. Mater. 2005, 17, 217–221. [Google Scholar] [CrossRef]
- Sarr, D.H.; Kazunga, C.; Charles, M.J.; Pavlovich, J.G.; Aitken, M.D. Decomposition of tetrachloro-1,4-benzoquinone (p-chloranil) in aqueous solution. Environ. Sci. Technol. 1995, 29, 2735–2740. [Google Scholar] [CrossRef]










| CPs | LOD (μM) | LR (μM) |
|---|---|---|
| 2,3,4-TCP | 0.3 | 0.3~100 |
| 2,4,6-TCP | 0.3 | 0.3~100 |
| 3,4,5-TCP | 0.07 | 0.1~100 |
| 2,4,5-TCP | 0.01 | 0.03~100 |
| 2,3,6-TCP | 0.07 | 0.07~100 |
| 2,3,5-TCP | 0.003 | 0.007~100 |
| 2,3,4,6-TeCP | 0.01 | 0.03~100 |
| 2,3,4,5-TeCP | 0.01 | 0.03~100 |
| 2,3,5,6-TeCP | 0.007 | 0.01~100 |
| PCP | 0.007 | 0.01~100 |
| XAr | LOD (μM) | LR (μM) |
|---|---|---|
| PCP | 0.01~70 | 0.007 |
| TCS | 0.07~30 | 0.03 |
| TBBPA | 0.1~10 | 0.07 |
| 2,3-DCNQ | 0.1~100 | 0.07 |
| PCB | 0.1~10 | 0.07 |
| PCB | 0.1~10 | 0.07 |
| T3 | 0.03~1 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, B.-Z.; Tang, M.; Huang, C.-H.; Mao, L. Detecting and Quantifying Polyhaloaromatic Environmental Pollutants by Chemiluminescence-Based Analytical Method. Molecules 2021, 26, 3365. https://doi.org/10.3390/molecules26113365
Zhu B-Z, Tang M, Huang C-H, Mao L. Detecting and Quantifying Polyhaloaromatic Environmental Pollutants by Chemiluminescence-Based Analytical Method. Molecules. 2021; 26(11):3365. https://doi.org/10.3390/molecules26113365
Chicago/Turabian StyleZhu, Ben-Zhan, Miao Tang, Chun-Hua Huang, and Li Mao. 2021. "Detecting and Quantifying Polyhaloaromatic Environmental Pollutants by Chemiluminescence-Based Analytical Method" Molecules 26, no. 11: 3365. https://doi.org/10.3390/molecules26113365
APA StyleZhu, B. -Z., Tang, M., Huang, C. -H., & Mao, L. (2021). Detecting and Quantifying Polyhaloaromatic Environmental Pollutants by Chemiluminescence-Based Analytical Method. Molecules, 26(11), 3365. https://doi.org/10.3390/molecules26113365