Betulin, a Newly Characterized Compound in Acacia auriculiformis Bark, Is a Multi-Target Protein Kinase Inhibitor
Abstract
:1. Introduction
2. Results
2.1. Purification of Betulin from Acacia Auriculiformis Stem Bark and Evaluation of Its Biological Activity against Disease-Related Protein Kinases
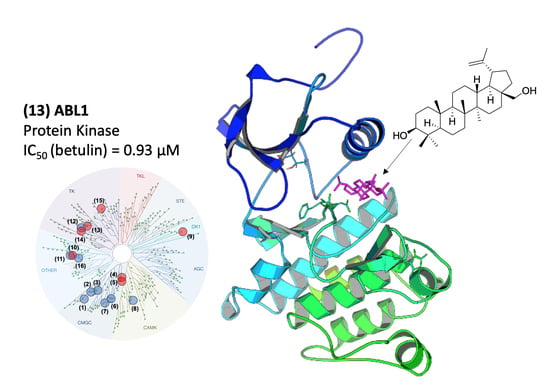
2.2. Molecular Mechanism of ABL1 Inhibition by Betulin
2.3. Molecular Modeling of the ABL1-Betulin Complex
2.4. Betulin Selectively Inhibits Proliferation of Human Leukemic Cells
2.5. Effects of Betulin on the MAPK/ERK Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Lines and Culture
4.3. Purification of Natural Products from Plant Material
4.4. Protein Kinase Assays
4.5. Molecular Docking
4.6. Drug Treatment and Cell Viability Assay
4.7. Immunoblot Analysis and Antibodies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rates, S.M. Plants as source of drugs. Toxicon 2001, 39, 603–613. [Google Scholar] [CrossRef]
- Raskin, I.; Ribnicky, D.M.; Komarnytsky, S.; Ilic, N.; Poulev, A.; Borisjuk, N.; Brinker, A.; Moreno, D.A.; Ripoll, C.; Yakoby, N.; et al. Plants and human health in the twenty-first century. Trends Biotechnol. 2002, 20, 522–531. [Google Scholar] [CrossRef]
- Siveen, K.S.; Prabhu, K.S.; Achkar, I.W.; Kuttikrishnan, S.; Shyam, S.; Khan, A.Q.; Merhi, M.; Dermime, S.; Uddin, S. Role of non receptor tyrosine kinases in hematological malignances and its targeting by natural products. Mol. Cancer 2018, 17, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, M.S. The role of natural product chemistry in drug discovery. J. Nat. Prod. 2004, 67, 2141–2153. [Google Scholar] [CrossRef] [PubMed]
- Ebada, S.S.; Edrada, R.A.; Lin, W.; Proksch, P. Methods for isolation, purification and structural elucidation of bioactive secondary metabolites from marine invertebrates. Nat. Protoc. 2008, 3, 1820–1831. [Google Scholar] [CrossRef] [PubMed]
- Manning, G. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capdeville, R.; Buchdunger, E.; Zimmermann, J.; Matter, A. Glivec (sti571, imatinib), a rationally developed, targeted anticancer drug. Nat. Rev. Drug Discov. 2002, 1, 493–502. [Google Scholar] [CrossRef]
- Nguyen, T.-N.-D.; Feizbakhsh, O.; Sfecci, E.; Baratte, B.; Delehouzé, C.; Garcia, A.; Moulin, C.; Colas, P.; Ruchaud, S.; Mehiri, M.; et al. Kinase-based screening of marine natural extracts leads to the identification of a cytotoxic high molecular weight metabolite from the mediterranean sponge crambe tailliezi. Mar. Drugs 2019, 17, 569. [Google Scholar] [CrossRef] [Green Version]
- Roskoski, R. Properties of fda-approved small molecule protein kinase inhibitors. Pharmacol. Res. 2019, 144, 19–50. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. Fda-Approved Small Molecule Protein Kinase Inhibitors; Blue Ridge Institute for Medical Research: Horse Shoe, NC, USA. Available online: http://www.brimr.org/PKI/PKIs.htm (accessed on 30 March 2021).
- Bournez, C.; Carles, F.; Peyrat, G.; Aci-Seche, S.; Bourg, S.; Meyer, C.; Bonnet, P. Comparative assessment of protein kinase inhibitors in public databases and in pkidb. Molecules 2020, 25, 3226. [Google Scholar] [CrossRef]
- Roskoski, R. Properties of fda-approved small molecule protein kinase inhibitors: A 2021 update. Pharmacol. Res. 2021, 165, 105463. [Google Scholar] [CrossRef] [PubMed]
- Guerra, B.; Issinger, O.-G. Natural compounds and derivatives as ser/thr protein kinase modulators and inhibitors. Pharmaceuticals 2019, 12, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadu, A.; Agunu, A.; Nguyen, T.-N.-D.; Baratte, B.; Foll-Josselin, B.; Ruchaud, S.; Serive, B.; Bach, S. Constituents of Acacia nilotica (L.) delile with novel kinase inhibitory activity. Planta Med. Int. Open 2017, 4, e108–e113. [Google Scholar] [CrossRef] [Green Version]
- Girijashankar, V. Micropropagation of multipurpose medicinal tree Acacia auriculiformis. Res. J. Med. Plant 2011, 5, 462–466. [Google Scholar]
- Kaur, K.; Arora, S.; Hawthorne, M.E.; Kaur, S.; Kumar, S.; Mehta, R.G. A correlative study on antimutagenic and chemopreventive activity of Acacia auriculiformisa. Cunn. Andacacia nilotica (L.) willd. Ex del. Drug Chem. Toxicol. 2002, 25, 39–64. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, Y.; Ono, K. Composition for Prevention and/or Treatment of Tumors Containing Acacia Bark Derivative. U.S. Patent 8,124,137B2, 28 February 2012. [Google Scholar]
- Mahato, S.B.; Pal, B.C.; Price, K.K. Structure of acaciaside, a triterpenoid trisaccharide from Acacia auriculiformis. Phytochemistry 1989, 28, 207–210. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Kumar, S.; Arora, S. Evaluation of antioxidant potential of ethyl acetate extract/fractions of Acacia auriculiformis a. Cunn. Food Chem. Toxicol. 2007, 45, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, S.; Kumar, S.; Arora, S. Free radical-scavenging activity of acetone extract/fractions of Acacia auriculiformis a. Cunn. Food Chem. 2007, 103, 1403–1410. [Google Scholar] [CrossRef]
- Garai, S.; Mahato, S.B. Isolation and structure elucidation of three triterpenoid saponins from Acacia auriculiformis. Phytochemistry 1997, 44, 137–140. [Google Scholar] [CrossRef]
- Mandal, P.; Sinha Babu, S.P.; Mandal, N.C. Antimicrobial activity of saponins from Acacia auriculiformis. Fitoterapia 2005, 76, 462–465. [Google Scholar] [CrossRef]
- Ahmadu, A.A.; Lawal, B.A.; Haruna, A.; Mustapha, L. Tetrahydroxy flavone from Acacia auriculiformis a. Cunn ex benth. (fabaceae) with novel kinase activity. Pharmacogn. J. 2019, 11, 559–563. [Google Scholar] [CrossRef] [Green Version]
- Barik, M.; Rawani, A.; Laskar, S.; Chandra, G. Evaluation of mosquito larvicidal activity of fruit extracts of Acacia auriculiformis against the japanese encephalitis vector culex vishnui. Nat. Prod. Res. 2018, 33, 1682–1686. [Google Scholar] [CrossRef]
- Prachayasittikul, S.; Saraban, P.; Cherdtrakulkiat, R.; Ruchirawat, S.; Prachayasittikul, V. New bioactive triterpenoids and antimalarial activity of diospyros rubra lec. EXCLI J. 2010, 9, 1–10. [Google Scholar]
- Qian, P.; Mu, X.-T.; Su, B.; Gao, L.; Zhang, D.-F. Identification of the anti-breast cancer targets of triterpenoids in liquidambaris fructus and the hints for its traditional applications. BMC Complement Altern. Med. 2020, 20, 369. [Google Scholar]
- Trott, O.; Olson, A.J. Autodock vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Nagar, B.; Bornmann, W.G.; Pellicena, P.; Schindler, T.; Veach, D.R.; Miller, W.T.; Clarkson, B.; Kuriyan, J. Crystal structures of the kinase domain of c-abl in complex with the small molecule inhibitors pd173955 and imatinib (sti-571). Cancer Res. 2002, 62, 4236–4243. [Google Scholar] [PubMed]
- Hevener, K.E.; Zhao, W.; Ball, D.M.; Babaoglu, K.; Qi, J.; White, S.W.; Lee, R.E. Validation of molecular docking programs for virtual screening against dihydropteroate synthase. J. Chem. Inf. Model. 2009, 49, 444–460. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, H.I.; Ozturk, S.E.; Ali, T.F.S.; Radwan, M.O.; Tateishi, H.; Koga, R.; Ocak, Z.; Can, M.; Otsuka, M.; Fujita, M. The first pentacyclic triterpenoid gypsogenin derivative exhibiting anti-abl1 kinase and anti-chronic myelogenous leukemia activities. Biol. Pharm. Bull. 2018, 41, 570–574. [Google Scholar] [CrossRef] [Green Version]
- Król, S.K.; Kiełbus, M.; Rivero-Müller, A.; Stepulak, A. Comprehensive review on betulin as a potent anticancer agent. BioMed Res. Int. 2015, 2015, 584189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heisterkamp, N.; Stam, K.; Groffen, J.; de Klein, A.; Grosveld, G. Structural organization of the bcr gene and its role in the ph’ translocation. Nature 1985, 315, 758–761. [Google Scholar] [CrossRef]
- McGahon, A.J.; Brown, D.G.; Martin, S.J.; Amarante-Mendes, G.P.; Cotter, T.G.; Cohen, G.M.; Green, D.R. Downregulation of bcr-abl in k562 cells restores susceptibility to apoptosis: Characterization of the apoptotic death. Cell Death Differ 1997, 4, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Šiman, P.; Filipová, A.; Tichá, A.; Niang, M.; Bezrouk, A.; Havelek, R. Effective method of purification of betulin from birch bark: The importance of its purity for scientific and medicinal use. PLoS ONE 2016, 11, e0154933. [Google Scholar] [CrossRef] [Green Version]
- Radwan, M.O.; Ismail, M.A.H.; El-Mekkawy, S.; Ismail, N.S.M.; Hanna, A.G. Synthesis and biological activity of new 18β-glycyrrhetinic acid derivatives. Arab. J. Chem. 2016, 9, 390–399. [Google Scholar] [CrossRef] [Green Version]
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Urban, M.; Sarek, J. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod. Rep. 2006, 23, 394–411. [Google Scholar] [CrossRef]
- Kirstgen, M.; Lowjaga, K.A.A.T.; Müller, S.F.; Goldmann, N.; Lehmann, F.; Alakurtti, S.; Yli-Kauhaluoma, J.; Glebe, D.; Geyer, J. Selective hepatitis b and d virus entry inhibitors from the group of pentacyclic lupane-type betulin-derived triterpenoids. Sci. Rep. 2020, 10, 21772. [Google Scholar] [CrossRef]
- Zhou, B.-N.; Johnson, R.K.; Mattern, M.R.; Fisher, P.W.; Kingston, D.G.I. The first naturally occurring tie2 kinase inhibitor. Org. Lett. 2001, 3, 4047–4049. [Google Scholar] [CrossRef] [PubMed]
- Hollosy, F.; Meszaros, G.; Bokonyi, G.; Idei, M.; Seprodi, A.; Szende, B.; Keri, G. Cytostatic, cytotoxic and protein tyrosine kinase inhibitory activity of ursolic acid in a431 human tumor cells. Anticancer Res. 2000, 20, 4563–4570. [Google Scholar] [PubMed]
- Roskoski, R. Properties of fda-approved small molecule protein kinase inhibitors: A 2020 update. Pharmacol. Res. 2020, 152, 104609. [Google Scholar] [CrossRef]
- Lehne, G. P-glycoprotein as a drug target in the treatment of multidrug resistant cancer. Curr. Drug Targets 2000, 1, 85–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, R.M.; Lee, K.; Kang, J.S.; Lee, C.W.; Lee, K.H.; Kim, J.H.; Yang, J.W.; Kim, B.G.; Han, G.; Park, S.-K.; et al. Kbh-a42, a histone deacetylase inhibitor, inhibits the growth of doxorubicin-resistant leukemia cells expressing p-glycoprotein. Oncol. Rep. 2010, 23, 801–809. [Google Scholar]
- Wang, H.M.; Soica, C.M.; Wenz, G. A comparison investigation on the solubilization of betulin and betulinic acid in cyclodextrin derivatives. Nat. Prod. Commun. 2012, 7, 289–291. [Google Scholar] [CrossRef] [Green Version]
- Jäger, S.; Laszczyk, M.; Scheffler, A. A preliminary pharmacokinetic study of betulin, the main pentacyclic triterpene from extract of outer bark of birch (betulae alba cortex). Molecules 2008, 13, 3224–3235. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, N.; Bonnet, P.; Brion, J.-D.; Peyrat, J.-F.; Bignon, J.; Levaique, H.; Josselin, B.; Robert, T.; Colas, P.; Bach, S.; et al. Identification of a new series of flavopiridol-like structures as kinase inhibitors with high cytotoxic potency. Eur. J. Med. Chem. 2020, 199, 112355. [Google Scholar] [CrossRef]
- DeLano, W.L. The Pymol User’s Manual; DeLano Scientific: San Carlos, CA, USA, 2002. [Google Scholar]
- Delehouzé, C.; Leverrier-Penna, S.; Le Cann, F.; Comte, A.; Jacquard-Fevai, M.; Delalande, O.; Desban, N.; Baratte, B.; Gallais, I.; Faurez, F.; et al. 6e11, a highly selective inhibitor of receptor-interacting protein kinase 1, protects cells against cold hypoxia-reoxygenation injury. Sci. Rep. 2017, 7, 12931. [Google Scholar] [CrossRef] [Green Version]
- Bach, S.; Knockaert, M.; Reinhardt, J.; Lozach, O.; Schmitt, S.; Baratte, B.; Koken, M.; Coburn, S.P.; Tang, L.; Jiang, T.; et al. Roscovitine targets, protein kinases and pyridoxal kinase. J. Biol. Chem. 2005, 280, 31208–31219. [Google Scholar] [CrossRef] [Green Version]






| Betulin 1 Tested at: | CDK5/ p25 | CDK9/ CyclinT | Haspin | Pim1 | GSK-3β | CK1ε | JAK3 | ABL1 |
|---|---|---|---|---|---|---|---|---|
| 10 µg/mL | 99 | 60 | 91 | 90 | 30 | 48 | 25 | 21 |
| 1 µg/mL | 94 | 88 | 72 | ≥100 | 38 | 53 | 28 | 36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadu, A.A.; Delehouzé, C.; Haruna, A.; Mustapha, L.; Lawal, B.A.; Udobre, A.; Baratte, B.; Triscornia, C.; Autret, A.; Robert, T.; et al. Betulin, a Newly Characterized Compound in Acacia auriculiformis Bark, Is a Multi-Target Protein Kinase Inhibitor. Molecules 2021, 26, 4599. https://doi.org/10.3390/molecules26154599
Ahmadu AA, Delehouzé C, Haruna A, Mustapha L, Lawal BA, Udobre A, Baratte B, Triscornia C, Autret A, Robert T, et al. Betulin, a Newly Characterized Compound in Acacia auriculiformis Bark, Is a Multi-Target Protein Kinase Inhibitor. Molecules. 2021; 26(15):4599. https://doi.org/10.3390/molecules26154599
Chicago/Turabian StyleAhmadu, Augustine A., Claire Delehouzé, Anas Haruna, Lukman Mustapha, Bilqis A. Lawal, Aniefiok Udobre, Blandine Baratte, Camilla Triscornia, Axelle Autret, Thomas Robert, and et al. 2021. "Betulin, a Newly Characterized Compound in Acacia auriculiformis Bark, Is a Multi-Target Protein Kinase Inhibitor" Molecules 26, no. 15: 4599. https://doi.org/10.3390/molecules26154599
APA StyleAhmadu, A. A., Delehouzé, C., Haruna, A., Mustapha, L., Lawal, B. A., Udobre, A., Baratte, B., Triscornia, C., Autret, A., Robert, T., Bulinski, J. C., Rousselot, M., Simoes Eugénio, M., Dimanche-Boitrel, M. -T., Petzer, J. P., Legoabe, L. J., & Bach, S. (2021). Betulin, a Newly Characterized Compound in Acacia auriculiformis Bark, Is a Multi-Target Protein Kinase Inhibitor. Molecules, 26(15), 4599. https://doi.org/10.3390/molecules26154599