Performance and Mechanism of Alkylimidazolium Ionic Liquids as Corrosion Inhibitors for Copper in Sulfuric Acid Solution
Abstract
:1. Introduction
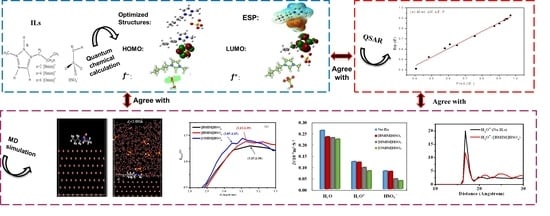
2. Results and Discussion
2.1. Structure and Reactivity of Three ILs
2.1.1. Optimized Geometry Structures and the Frontier Molecule Orbital Distribution
2.1.2. Global Reactivity for Fur ILs in Gas Phase
2.1.3. Local Reactivity
2.1.4. Electrostatic Potential (ESP) Diagrams
2.1.5. Reactive Parameters of Three ILs in Solution
2.2. Molecular Dynamics (MD) Simulation
2.3. QSAR of Reactive Parameters and the Inhibition Efficiency of Three ILs
3. Materials and Methods
3.1. Geometry Structure Optimization and the Reactivity of ILs
3.2. Adsorption of ILs on Copper Surface and the Inhibition Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Q.B.; Hua, Y.X. Effect of alk, ylimidazolium ionic liquids on the corrosion inhibition of copper in sulfuric acid solution. Acta Phys.-Chim. Sin. 2011, 27, 655–663. [Google Scholar]
- Fateh, A.; Aliofkhazraei, M.; Rezvanian, A.R. Review of corrosive environments for copper and its corrosion inhibitors. Arabian J. Chem. 2020, 13, 481–544. [Google Scholar] [CrossRef]
- Antonijevic, M.M.; Petrovic, M.B. Copper corrosion inhibitors. A review. Int. J. Electrochem. Sci. 2008, 3, 1–28. [Google Scholar]
- Mihajlović, M.B.P.; Antonijević, M.M. Copper corrosion inhibitors. Period 2008–2014. A review. Int. J. Electrochem. Sci. 2015, 10, 1027–1053. [Google Scholar]
- El-Katori, E.E.; Abousalem, A.S. Impact of some pyrrolidinium ionic liquids on copper dissolution behavior in acidic environment, experimental, morphological and theoretical insights. RSC Adv. 2019, 9, 20760–20777. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Zhang, S.; Lu, Y.; Tan, B.; Chen, S.; Guo, L. Synergistic corrosion inhibition effect of thiazolyl-based ionic liquids between anions and cations for copper in HCl solution. Appl. Surf. Sci. 2019, 483, 901–911. [Google Scholar]
- Qiang, Y.; Zhang, S.; Guo, L.; Zheng, X.; Xiang, B.; Chen, S. Experimental and theoretical studies of four allyl imidazolium-based ionic liquids as green inhibitors for copper corrosion in sulfuric acid. Corros. Sci. 2017, 119, 68–78. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Bahadur, I.; Quraishi, M.A. An overview on plant extracts as environmental sustainable and green corrosion inhibitors for metals and alloys in aggressive corrosive media. J. Mol. Liq. 2018, 266, 577–590. [Google Scholar] [CrossRef]
- Verma, C.; Lgaz, H.; Verma, D.K.; Eno, E.E.; Bahadur, I.; Quraishi, M.A. Molecular dynamics and Monte Carlo simulations as powerful tools for study of interfacial adsorption behavior of corrosion inhibitors in aqueous phase, a review. J. Mol. Liq. 2018, 260, 99–120. [Google Scholar] [CrossRef]
- Verma, C.; Olasunkanmi, L.O.; Ebenso, E.E.; Quraishi, M.A. Substituents effect on corrosion inhibition performance of organic compounds in aggressive ionic solutions, a review. J. Mol. Liq. 2018, 251, 100–118. [Google Scholar] [CrossRef]
- Nandi, M.M.; Banerjee, R. Organic corrosion inhibitors for copper and brass—A review. J. Indian Chem. Soc. 2014, 91, 977–989. [Google Scholar]
- Yang, H.M. Role of Organic and Eco-Friendly Inhibitors on the Corrosion Mitigation of Steel in Acidic Environments—A State-of-Art Review. Molecules 2021, 26, 3473. [Google Scholar] [CrossRef]
- Hu, J.; He, S.; Zhang, Y.; Ma, H.; Zhang, X.; Chen, X. Theoretical Insights into the Solvent Polarity Effect on the Quality of Self-Assembled N-Octadecanethiol Monolayers on Cu (111) Surfaces. Molecules 2018, 23, 733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dzyuba, S.V.; Bartsch, R.A. Influence of structural variations in 1-alkyl (aralkyl)-3-methylimidazolium hexafluorophosphates and bis (trifluoromethylsulfonyl) imides on physical properties of the ionic liquids. ChemPhysChem 2002, 3, 161–166. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A. Ionic liquids as green and sustainable corrosion inhibitors for metals and alloys: An overview. J. Mol. Liq. 2017, 233, 403–414. [Google Scholar] [CrossRef]
- Verma, C.; Alrefaee, S.H.; Quraishi, M.A.; Ebenso, E.E.; Hussain, C.M. Recent developments in sustainable corrosion inhibitors using ionic liquids: A review. J. Mol. Liq. 2020, 114484. [Google Scholar]
- Arellanes-Lozada, P.; Olivares-Xometl, O.; Guzmán-Lucero, D.; Likhanova, N.V.; Domínguez-Aguilar, M.A.; Lijanova, I.V.; Arce-Estrada, E. The inhibition of aluminum corrosion in sulfuric acid by poly (1-vinyl-3-alkyl-imidazolium hexafluorophosphate). Materials 2014, 7, 5711–5734. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Huang, A.; Lin, D.; Talha, M.; Liu, H.; Lin, Y. Imidazolium-based ionic liquid as efficient corrosion inhibitor for AA6061 alloy in HCl solution. Materials 2020, 13, 4672. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, T.; Sanes, J.; Jiménez, A.E.; Bermúdez, M.D. Surface interactions, corrosion processes and lubricating performance of protic and aprotic ionic liquids with OFHC copper. Appl. Surf. Sci. 2013, 273, 578–597. [Google Scholar] [CrossRef]
- Allal, H.; Belhocine, Y.; Zouaoui, E. Computational study of some thiophene derivatives as aluminium corrosion inhibitors. J. Mol. Liq. 2018, 265, 668–678. [Google Scholar] [CrossRef]
- Kaya, S.; Tüzün, B.; Kaya, C.; Obot, I.B. Determination of corrosion inhibition effects of amino acids, Quantum chemical and molecular dynamic simulation study. J. Taiwan Inst. Chem. Eng. 2016, 58, 528–535. [Google Scholar] [CrossRef]
- Yesudass, S.; Olasunkanmi, L.O.; Bahadur, I.; Kabanda, M.M.; Obot, I.B.; Ebenso, E.E. Experimental and theoretical studies on some selected ionic liquids with different cations/anions as corrosion. inhibitors for mild steel in acidic medium. J. Taiwan Inst. Chem. Eng. 2016, 64, 252–268. [Google Scholar] [CrossRef]
- Murulana, L.C.; Singh, A.K.; Shukla, S.K.; Kabanda, M.M.; Ebenso, E.E. Experimental and quantum chemical studies of some bis (trifluoromethyl-sulfonyl) imide imidazolium-based ionic liquids as corrosion inhibitors for mild steel in hydrochloric acid solution. Ind. Eng. Chem. Res. 2012, 51, 13282–13299. [Google Scholar] [CrossRef]
- Shahraki, M.; Dehdab, M.; Elmi, S. Theoretical studies on the corrosion inhibition performance of three amine derivatives on carbon steel, molecular dynamics simulation and density functional theory approaches. J. Taiwan Inst. Chem. Eng. 2016, 62, 313–321. [Google Scholar] [CrossRef]
- Kaya, S.; Kaya, C.; Guo,, L.; Kandemirli, F.; Tüzün, B.; Uğurlu, İ.; Saraçoğlu, M. Quantum chemical and molecular dynamics simulation studies on inhibition performances of some thiazole and thiadiazole derivatives against corrosion of iron. J. Mol. Liq. 2016, 219, 497–504. [Google Scholar] [CrossRef]
- Martinez, S. Inhibitory mechanism of mimosa tannin using molecular modeling and substitutional adsorption isotherms. Mater. Chem. Phys. 2003, 77, 97–102. [Google Scholar]
- Tian, G.; Yuan, K. Adsorption and inhibition behavior of imidazolium tetrafluoroborate derivatives as green corrosion inhibitors for carbon steel. J. Mol. Model. 2021, 27, 1–16. [Google Scholar] [CrossRef]
- Daoud, D.; Douadi, T.; Hamani, H.; Chafaa, S.; Al-Noaimi, M. Corrosion inhibition of mild steel by two new S-heterocyclic compounds in 1 M HCl: Experimental and computational study. Corros. Sci. 2015, 94, 21–37. [Google Scholar] [CrossRef]
- Fukui, K. Role of frontier orbitals in chemical reactions. Science 1982, 218, 747–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabanda, M.M.; Ebenso, E.E. Density functional theory and quantitative structure-activity relationship studies of some quinoxaline derivatives as potential corrosion inhibitors for copper in acidic medium. Int. J. Electrochem. Sci. 2012, 7, 8713–8733. [Google Scholar]
- Saha, S.K.; Banerjee, P.A. Theoretical approach to understand the inhibition mechanism of steel corrosion with two aminobenzonitrile inhibitors. RSC Adv. 2015, 5, 71120–71130. [Google Scholar] [CrossRef]
- Peljhan, S.; Kokalj, A. DFT study of gas-phase adsorption of benzotriazole on Cu (111), Cu (100), Cu (110), and low coordinated defects thereon. Phys. Chem. Chem. Phys. 2011, 13, 20408–20417. [Google Scholar] [CrossRef]
- Shi, X.; Jiang, Y.; Wan, H.; Hao, Y.; Chen, S.; Li, C.; Sun, S.; Hu, S. Density functional theory analysis on four pyrazine corrosion inhibitors and their adsorption behavior on Cu (111) surface. CIESC J. 2017, 68, 3211–3217. [Google Scholar]
- Ma, Y.; Han, F.; Li, Z.; Xia, C. Corrosion behavior of metallic materials in acidic-functionalized ionic liquids. ACS Sustain. Chem. Eng. 2016, 4, 633–639. [Google Scholar] [CrossRef]
- Zeng, J.; Shi, W.; Sun, G.; Chen, S. Molecular dynamics simulation of the interaction between benzotriazole and its derivatives and Cu2O crystal. J. Mol. Liq. 2016, 223, 150–155. [Google Scholar] [CrossRef]
- Musa, A.Y.; Kadhum, A.A.H.; Mohamad, A.B.; Rahoma, A.A.B.; Mesmari, H. Electrochemical and quantum chemical calculations on 4, 4-dimethyloxazolidine-2-thione as inhibitor for mild steel corrosion in hydrochloric acid. J. Mol. Struct. 2010, 969, 233–237. [Google Scholar] [CrossRef]
- Liu, J.; Cao, D.; Zhang, L. Molecular dynamics study on nanoparticle diffusion in polymer melts: A test of the Stokes–Einstein law. J. Phys. Chem. C 2008, 112, 6653–6661. [Google Scholar] [CrossRef]
- Frisch, M.J.E.A.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.; et al. Gaussian 09 Revision A, 3rd ed.; Gaussian, Inc.: Wallingford, UK, 2009. [Google Scholar]
- Parr, R.G. Density Functional Theory of Atoms and Molecules, in Horizons of Quantum Chemistry; Springer International Publishing: Dordrecht, The Netherlands, 1980; pp. 5–15. [Google Scholar]
- Tian, G.; Zhou, W. Theoretical Study of the Structure and Property of Ionic Liquids as Corrosion Inhibitor, in Density Functional Theory Calculations; IntechOpen: London, UK, 2020. [Google Scholar]
- Garcia, G.; Atilhan, M.; Aparicio, S. A density functional theory insight towards the rational design of ionic liquids for SO2 capture. Phys. Chem. Chem. Phys. 2015, 17, 13559–13574. [Google Scholar] [CrossRef] [Green Version]
- Pareek, S.; Jain, D.; Hussain, S. A new insight into corrosion inhibition mechanism of copper in aerated 3.5 wt.% NaCl solution by eco-friendly Imidazopyrimidine Dye, experimental and theoretical approach. Chem. Eng. J. 2019, 358, 725–742. [Google Scholar] [CrossRef]
- Ju, H.; Kai, Z.P.; Li, Y. Aminic nitrogen-bearing polydentate Schiff base compounds as corrosion inhibitors for iron in acidic media, a quantum chemical calculation. Corros. Sci. 2008, 50, 865–871. [Google Scholar] [CrossRef]
- Albrakaty, R.H.; Wazzan, N.A.; Obot, I.B. Theoretical study of the mechanism of corrosion inhibition of carbon steel in acidic solution by 2-aminobenzothaizole and 2-mercatobenzothiazole. Int. J. Electrochem. Sci. 2018, 13, 3535–3554. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn, a multifunctional wavefunction analyzer. J. Comp. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhu, S.; Zhang, S.; Li, W. Theoretical studies of three triazole derivatives as corrosion inhibitors for mild steel in acidic medium. Corros. Sci. 2014, 87, 366–375. [Google Scholar] [CrossRef]
- Lukovits, I.; Bakó, I.; Shaban, A.; Kálmán, E. Polynomial model of the inhibition mechanism of thiourea derivatives. Electrochim. Act. 1998, 43, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Salarvand, Z.; Amirnasr, M.; Talebian, M.; Raeissi, K.; Meghdadi, S. Enhanced corrosion resistance of mild steel in 1 M HCl solution by trace amount of 2-phenyl-benzothiazole derivatives, Experimental, quantum chemical calculations and molecular dynamics (MD) simulation studies. Corros. Sci. 2017, 114, 133–145. [Google Scholar] [CrossRef]
- Awad, M.K.; Mustafa, M.R.; Elnga, M.M.A. Computational simulation of the molecular structure of some triazoles as inhibitors for the corrosion of metal surface. J. Mol. Struct. Theochem. 2010, 959, 66–74. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, X.; Ji, L.; Hu, H.; Li, Q. Quantitative structure–activity relationship model for amino acids as corrosion inhibitors based on the support vector machine and molecular design. Corros. Sci. 2014, 83, 261–271. [Google Scholar] [CrossRef]
- Materials Studio, Version 6.0; Accelrys Software Inc.: San Diego, CA, USA, 2012; Available online: http://accelrys.com/products/materials-studio/index.html (accessed on 23 July 2021).
- Saha, S.K.; Ghosh, P.; Hens, A.; Raeissi, K.; Meghdadi, S. Density functional theory and molecular dynamics simulation study on corrosion inhibition performance of mild steel by mercapto-quinoline Schiff base corrosion inhibitor. Physica E 2015, 66, 332–341. [Google Scholar] [CrossRef]
- Xie, S.W.; Liu, Z.; Han, G.C.; Li, W.; Liu, J.; Chen, Z. Molecular dynamics simulation of inhibition mechanism of 3,5-dibromo salicylaldehyde Schiff’s base. Comput. Theor. Chem. 2015, 1063, 50–62. [Google Scholar] [CrossRef]











| [BMIM]HSO4 | [HMIM]HSO4 | [OMIM]HSO4 | |
|---|---|---|---|
| Etotal (au) | −1123.1 | −1201.7 | −1280.3 |
| EHOMO (ev) | −6.3138 | −6.3064 | −6.2289 |
| ELUMO (ev) | −1.3271 | −1.3260 | −1.2664 |
| ΔE (ev) | 4.9867 | 4.9804 | 4.9625 |
| 12.814 | 12.620 | 10.905 | |
| P | 144.54 | 169.69 | 193.14 |
| χ (ev) | 3.8205 | 3.8162 | 3.7477 |
| η (ev) | 2.4934 | 2.4902 | 2.4813 |
| σ (ev) | 0.4011 | 0.4016 | 0.4030 |
| ΔΝ | 0.2024 | 0.2036 | 0.2181 |
| Ι (ev) | 1.3271 | 1.3260 | 1.2664 |
| A (ev) | 6.3138 | 6.3064 | 6.2289 |
| ω (ev) | 2.9270 | 2.9241 | 2.8302 |
| MV (cm3/mol) | 161.45 | 199.07 | 217.35 |
| [BMIM]HSO4 | [HMIM]HSO4 | [OMIM]HSO4 | |
|---|---|---|---|
| Etotal (au) | −1123.1 | −1201.8 | −1280.4 |
| EHOMO (ev) | −7.3692 | −7.3527 | −7.3236 |
| ELUMO (ev) | −0.6825 | −0.6669 | −0.6457 |
| ΔE (ev) | 6.6867 | 6.6858 | 6.6779 |
| µ (D) | 27.236 | 23.473 | 22.116 |
| χ (ev) | 4.0259 | 4.0098 | 3.9847 |
| η (ev) | 3.3434 | 3.3429 | 3.3390 |
| σ (ev) | 0.2991 | 0.2991 | 0.2995 |
| ΔΝ | 0.4448 | 0.4472 | 0.4515 |
| Ι (ev) | 7.3603 | 7.3527 | 7.3236 |
| A (ev) | 0.6789 | 0.6669 | 0.6457 |
| ω (ev) | 2.4238 | 2.4049 | 2.3776 |
| P | 191.26 | 225.373 | 260.28 |
| ILs | [BMIM]HSO4 | [HMIM]HSO4 | [OMIM]HSO4 |
|---|---|---|---|
| Eadsorptio | −58.737 | −69.866 | −81.471 |
| ILs | [BMIM]HSO4 | [HMIM]HSO4 | [OMIM]HSO4 |
|---|---|---|---|
| Eadsorption | −59.080 | −71.154 | −83.134 |
| Atom | Number | Charge |
|---|---|---|
| Cu | 1 | −0.11 |
| Cu | 2 | −0.11 |
| Cu | 3 | −0.11 |
| Cu | 4 | −0.11 |
| Cu | 5 | 0.22 |
| Cu | 6 | 0.22 |
| Cu | 7 | 0.22 |
| Cu | 8 | 0.22 |
| Cu | 9 | −0.11 |
| Cu | 10 | −0.11 |
| Cu | 11 | −0.11 |
| Cu | 12 | −0.11 |
| Atom | [BMIM]HSO4 | [HMIM]HSO4 | [OMIM]HSO4 |
|---|---|---|---|
| C2 | −0.6686 | −0.5758 | −0.8694 |
| C1 | 0.0184 | 0.0032 | 0.0701 |
| C3 | −0.0320 | −0.0225 | 0.1357 |
| N4 | −0.0331 | −0.0216 | 0.2111 |
| H5 | 0.1643 | 0.1629 | 0.1722 |
| H6 | 0.1476 | 0.1453 | 0.1593 |
| N7 | 0.0631 | 0.0570 | 0.1440 |
| C14 | −0.2246 | −0.2202 | −0.2341 |
| H15 H16 | 0.2363 0.1817 | 0.2328 0.1820 | 0.2298 0.1793 |
| H17 | 0.1833 | 0.1851 | 0.1964 |
| H21 | 0.5641 | 0.5094 | 0.2502 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, G.; Yuan, K. Performance and Mechanism of Alkylimidazolium Ionic Liquids as Corrosion Inhibitors for Copper in Sulfuric Acid Solution. Molecules 2021, 26, 4910. https://doi.org/10.3390/molecules26164910
Tian G, Yuan K. Performance and Mechanism of Alkylimidazolium Ionic Liquids as Corrosion Inhibitors for Copper in Sulfuric Acid Solution. Molecules. 2021; 26(16):4910. https://doi.org/10.3390/molecules26164910
Chicago/Turabian StyleTian, Guocai, and Kaitao Yuan. 2021. "Performance and Mechanism of Alkylimidazolium Ionic Liquids as Corrosion Inhibitors for Copper in Sulfuric Acid Solution" Molecules 26, no. 16: 4910. https://doi.org/10.3390/molecules26164910
APA StyleTian, G., & Yuan, K. (2021). Performance and Mechanism of Alkylimidazolium Ionic Liquids as Corrosion Inhibitors for Copper in Sulfuric Acid Solution. Molecules, 26(16), 4910. https://doi.org/10.3390/molecules26164910