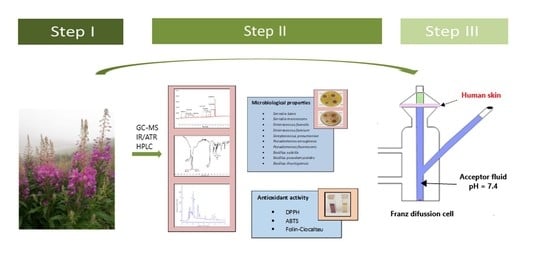
In Vitro Human Skin Penetration, Antioxidant and Antimicrobial Activity of Ethanol-Water Extract of Fireweed (Epilobium angustifolium L.)
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of the FEE and Its Antioxidant Activity
2.2. Figures, Tables, and Schemes
2.3. Microbiological Assay
2.4. Skin Penetration
3. Discussion
3.1. Chemical Characterization of the FEE and Its Antioxidant Capacity
3.2. Microbiological Assay
3.3. Skin Penetration
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material
4.3. GC-MS and HPLC Analysis
4.4. Evaluation of the Antioxidant Capacity Using DPPH, ABTS, and Folin–Ciocalteu Methods
4.5. Microbiological Analysis
4.6. In Vitro Skin Permeation Studies of the FEE
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| FEE | fireweed ethanol-water extracts |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| TPTZ | 2,4,6-tripyridyl-s-triazine |
| GA | gallic acid |
| ChA | chlorogenic acid |
| 3,4-DHB | 3,4-dihydroxybenzoic acid |
| 4-HB | 4-hydroxybenzoic acid |
| CA | caffeic acid |
| PhA | phenolic acids |
| GC-MS | gas chromatography coupled with mass spectrometry |
| HPLC | high-performance liquid chromatography |
| TSA | tryptic-soya agar |
| TSB | liquid tryptone-soybean |
| TEAC | trolox equivalent antioxidant capacity |
| GAE | gallic acid equivalents |
| ROS | reactive oxygen species |
References
- Sõukand, R.; Mattalia, G.; Kolosova, V.; Stryamets, N.; Prakofjewa, J.; Belichenko, O.; Kuznetsova; Minuzzi, S.; Keedus, L.; Prūse, B.; et al. Inventing a herbal tradition: The complex roots of the current popularity of Epilobium angustifolium in Eastern Europe. J. Ethnopharmacol. 2020, 247, 112254. [Google Scholar] [CrossRef] [PubMed]
- Kalle, R.; Belichenko, O.; Kuznetsova, N.; Kolosova, V.; Prakofjewa, J.; Stryamets, N.; Mattalia, G.; Šarka, P.; Simanova, A.; Prūse, B.; et al. Gaining momentum: Popularization of Epilobium angustifolium as food and recreational tea on the Eastern edge of Europe. Appetite 2020, 150, 104638. [Google Scholar] [CrossRef] [PubMed]
- Lasinskas, M.; Jariene, E.; Vaitkeviciene, N.; Hallmann, E.; Najman, K. Effect of different durations of solid-phase fermentation for fireweed (Chamerion angustifolium (L.) Holub) leaves on the content of polyphenols and antioxidant activity in vitro. Molecules 2020, 25, 1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak, A.; Klimowicz, A.; Duchnik, W.; Kucharski, Ł.; Florkowska, K.; Muzykiewicz, A.; Wira, D.; Zielonka-Brzezicka, J.; Siedłowska, A.; Nadarzewska, K. Application of green-extraction technique to evaluate of antioxidative capacity of wild population of fireweed (Epilobium angustifolium). Herba Pol. 2019, 65, 18–30. [Google Scholar] [CrossRef]
- Battinelli, L.; Tita, B.; Evandri, M.G.; Mazzanti, G. Antimicrobial activity of Epilobium spp. extracts. Il. Farm 2001, 56, 345–348. [Google Scholar] [CrossRef]
- Karakaya, S.; Süntar, I.; Yakinci, O.F.; Sytar, O.; Ceribasi, S.; Dursunoglu, B.; Ozbek, H.; Guvenalp, Z. In vivo bioactivity assessment on Epilobium species: A particular focus on Epilobium angustifolium and its components on enzymes connected with the healing process. J. Ethnopharmacol. 2020, 262, 113207. [Google Scholar] [CrossRef]
- Adamska-Szewczyk, A.; Zgórka, G. Plant polyphenols in cosmetics—A review. Eur. J. Med. Technol. 2019, 3, 1–10. [Google Scholar]
- Liu, J.; Du, C.; Beaman, H.T.; Monroe, M.B.B. Characterization of Phenolic Acid Antimicrobial and Antioxidant Structure–Property Relationships. Pharmacy 2020, 12, 419. [Google Scholar] [CrossRef]
- Bertges, F.S.; Amaral, M.; Pereira Rodarte, M.; Fonseca, M.J.V.; Sousa, O.V.; Pinto Vilela, F.M.; Alves, M.S. Assessment of chemical changes and skin penetration of green Arabica coffee beans biotransformed by Aspergillus oryzae. Biocatal. Agric. Biotechnol. 2020, 23, 101512. [Google Scholar] [CrossRef]
- Esposito, S.; De Simone, G.; Pan, A.; Brambilla, P.; Gattuso, G.; Mastroianni, C.; Kertusha, B.; Contini, C.; Massoli, L.; Francisci, D.; et al. Epidemiology and Microbiology of Skin and Soft Tissue Infections: Preliminary Results of a National Registry. J. Chemother. 2018, 31, 9–14. [Google Scholar] [CrossRef]
- Chakraborty, S.P.; Pramanik, P.; Roy, S. Staphylococcus aureus Infection Induced Oxidative Imbalance in Neutrophils: Possible Protective Role of Nanoconjugated Vancomycin. ISRN Pharmacology 2012, 2012, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, S.P.; Roy, S.; Wiwanitkit, V. In vitro Staphylococcus aureus–induced oxidative stress in mice murine peritoneal macrophages: A duration–dependent approach. Asian Pac. J. Trop. Biomed. 2014, 4, S298–S304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okayama, Y. Oxidative Stress in Allergic and Inflammatory Skin Diseases. Curr. Drug Target Inflamm. Allergy 2005, 4, 517–519. [Google Scholar] [CrossRef] [PubMed]
- Pitz, H.S.; Pereira, A.; Blasius, M.B.; Voytena, A.P.L.; Affonso, R.C.L.; Fanan, S.; Trevisan, A.C.D.; Ribeiro-Do-Valle, R.M.; Maraschin, M. In Vitro Evaluation of the Antioxidant Activity and Wound Healing Properties of Jaboticaba (Plinia peruviana) Fruit Peel Hydroalcoholic Extract. Oxidative Med. Cell. Longev. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novak-Babič, M.; Gostinčar, C.; Gunde-Cimerman, N. Microorganisms populating the water-related indoor biome. Appl. Microbiol. Biotechnol. 2020, 104, 6443–6462. [Google Scholar] [CrossRef] [PubMed]
- Amparo, T.R.; Seibert, J.B.; Vieira, P.M.A.; Teixeira, L.F.M.; Dos Santos, O.D.; De Souza, G.H.B. Herbal medicines to the treatment of skin and soft tissue infections: Advantages of the multi-targets action. Phytother. Res. 2019, 34, 94–103. [Google Scholar] [CrossRef]
- Bisht, R.; Chanyal, S.; Agrawal, P.K. Antimicrobial and phytochemical analysis of leaf extract of medicinal fruit plants. Asian J. Pharm. Clin. Res. 2016, 9, 131–136. [Google Scholar]
- Pirvu, L.; Nicorescu, I.; Hlevca, C.; Albu, B.; Nicorescu, V. Epilobi Hirsuti Herba Extracts Influence the In Vitro Activity of Common Antibiotics on Standard Bacteria. Open Chem. 2016, 14, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Nazir, A.; Malik, K.; Qamar, H.; Basit, M.H.; Liaqat, A.; Shahid, M.; Khan, M.I.; Fatima, A.; Irshad, A.; Sadia, H. A review: Use of plant extracts and their phytochemical constituents to control antibiotic resistance in S. aureus. Pure Appl. Biol. 2020, 9, 720–727. [Google Scholar] [CrossRef]
- Nicolai, M.; Mota, J.; Fernandes, A.F.C.; Pereira, F.; Pereira, P.; Reis, C.P.; Velasco, M.V.R.; Baby, A.R.; Rosado, C.F.; Rijo, P. Assessment of the Potential Skin Application of Plectranthus ecklonii Benth. Pharmacy 2020, 13, 120. [Google Scholar] [CrossRef]
- Zgoda, J.R.; Porter, J.R. A convenient microdilution method for screening natural products against bacteria and fungi. Pharm. Biology 2001, 39, 221–225. [Google Scholar] [CrossRef]
- Vitalone, A.; Allkanjari, O. Epilobium spp.: Pharmacology and Phytochemistry. Phytother. Res. 2018, 32, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Monschein, M.; Jaindl, K.; Buzimkić, S.; Bucar, F. Content of phenolic compounds in wild populations of Epilobium angustifolium growing at different altitudes. Pharm. Biol. 2015, 53, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadam, P.; Patil, M.; Yadav, K. A Review on Phytopharmacopial Potential of Epilobium angustifolium. Pharmacogn. J. 2018, 10, 1076–1078. [Google Scholar] [CrossRef] [Green Version]
- Ferrante, C.; Chiavaroli, A.; Angelini, P.; Venanzoni, R.; Flores, G.A.; Brunetti, L.; Petrucci, M.; Politi, M.; Menghini, L.; Leone, S.; et al. Phenolic Content and Antimicrobial and Anti-Inflammatory Effects of Solidago virga-aurea, Phyllanthus niruri, Epilobium angustifolium, Peumus boldus, and Ononis spinosa Extracts. Antibiotics 2020, 9, 783. [Google Scholar] [CrossRef]
- Stajner, D.; Popović, B.M.; Boža, P. Evaluation of willow herb’s (Epilobium angustofolium L.) antioxidant and radical scavenging capacities. Phytother. Res. 2007, 21, 1242–1245. [Google Scholar] [CrossRef] [PubMed]
- Shikov, A.N.; Poltanov, E.A.; Dorman, H.J.D.; Makarov, V.G.; Tikhonov, V.P.; Hiltunen, R. Chemical Composition and in Vitro Antioxidant Evaluation of Commercial Water-Soluble Willow Herb (Epilobium angustifolium L.) Extracts. J. Agric. Food Chem. 2006, 54, 3617–3624. [Google Scholar] [CrossRef]
- Cando, D.; Morcuende, D.; Utrera, M.; Estévez, M. Phenolic-rich extracts from Willowherb (Epilobium hirsutum L.) inhibit lipid oxidation but accelerate protein carbonylation and discoloration of beef patties. Eur. Food Res. Technol. 2014, 238, 741–751. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Stankevičius, M.; Drevinskas, T.; Akuneca, I.; Kaškonas, P.; Bimbiraitė-Survilienė, K.; Maruška, A.; Ragažinskienė, O.; Kornyšova, O.; Briedis, V.; et al. Evaluation of phytochemical composition of fresh and dried raw material of introduced Chamerion angustifolium L. using chromatographic, spectrophotometric and chemometric techniques. Phytochemistry 2015, 115, 184–193. [Google Scholar] [CrossRef]
- Dzhafar, S.S.; Dalar, A.; Mükemre, M.; Ekin, S.; Yildiz, D.; Yunusoğlu, O. Phytochemical Profile and in vitro and in vivo Anticonvulsant and Antioxidant Activities of Epilobium hirsutum. Int. J. Second. Metab. 2020, 7, 63–76. [Google Scholar] [CrossRef]
- Kosalec, I.; Zovko, M.; Sankovic, K.; Kremer, D.; Pepeljnjak, S. Antioxidant and antimicrobial activity of willow herb (Epilobium angustifolium L.). Planta Med. 2008, 74, PA43. [Google Scholar] [CrossRef]
- Maruška, A.; Ragažinskienė, O.; Vyšniauskas, O.; Kaškonienė, V.; Bartkuvienė, V.; Kornysova, O.; Briedis, V.; Ramanauskienė, K. Flavonoids of willow herb (Chamerion angustifolium (L.) Holub) and their radical scavenging activity during vegetation. Adv. Med. Sci. 2014, 59, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Jürgenson, S.; Matto, V.; Raal, A. Vegetational variation of phenolic compounds in Epilobium angustifolium. Nat. Prod. Res. 2012, 26, 1951–1953. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.Y.; Wu, J.; Lin, P. Chemical Composition and Antimicrobial Activity of the Essential Oil from Epilobium angustifolium. Chem. Nat. Compd. 2016, 52, 1113–1115. [Google Scholar] [CrossRef]
- Bajer, T.; Šilhab, D.; Ventura, K.; Bajerová, P. Composition and antimicrobial activity of the essential oil, distilled aromatic water and herbal infusion from Epilobium parviflorum Schreb. Ind. Crops Prod. 2017, 100, 95–105. [Google Scholar] [CrossRef]
- Canlı, K.; Yetgin, A.; Akata, I.; Altuner, E.M. Antimicrobial Activity and Chemical Composition Screening of Epilobium montanum Root. Indian J. Pharm. Educ. Res. 2017, 51, s239–s243. [Google Scholar] [CrossRef]
- Adamczak, A.; Dreger, M.; Seidler-Łożykowska, K.; Wielgus, K. Fireweed (Epilobium angustifolium L.): Botany, phytochemistry and traditional uses. A review. Herba Pol. 2019, 65, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Sabulal, B.; Dan, M.; John, A.; Kurup, R.; Pradeep, N.S.; Valsamma, R.K.; George, V. Caryophyllene-rich rhizome oil of Zingiber nimmonii from South India: Chemical characterization and antimicrobial activity. Phytochemistry 2006, 67, 2469–2473. [Google Scholar] [CrossRef]
- Martínez-Pabón, M.C.; Ortega-Cuadros, M. Thymol, menthol and eucalyptol as agents for microbiological control in the oral cavity: A scoping review. Rev. Colomb. Cienc. Quím. Farm. 2020, 49, 44–69. [Google Scholar] [CrossRef]
- Prakash, A.; Vadivel, V.; Rubini, D.; Nithyanand, P. Antibacterial and antibiofilm activities of linalool nanoemulsions against Salmonella Typhimurium. Food Biosci. 2019, 28, 57–65. [Google Scholar] [CrossRef]
- Ruszová, E.; Cheel, J.; Pávek, S.; Moravcová, M.; Hermannová, M.; Matějková, I.; Spilková, J.; Velebný, V.; Kubala, L. Epilobium angustifolium extract demonstrates multiple effects on dermal fibroblasts in vitro and skin photo-protection in vivo. Gen. Physiol. Biophys. 2013, 32, 347–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remmel, I.; Vares, L.; Toom, L.; Matto, V.; Raal, A. Phenolic Compounds in Five Epilobium Species Collected from Estonia. Nat. Prod. Commun. 2012, 7, 1323–1324. [Google Scholar] [CrossRef] [Green Version]
- Wojdylo, A.; Oszmianski, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Ramstead, A.G.; Kirpotina, L.N.; Voyich, J.M.; Jutila, M.A.; Quinn, M.T. Therapeutic Potential of Polyphenols from Epilobium Angustifolium (Fireweed). Phytother. Res. 2016, 30, 1287–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granica, S.; Piwowarski, J.P.; Czerwińska, M.E.; Kiss, A.K. Phytochemistry, pharmacology and traditional uses of different Epilobium species (Onagraceae): A review. J. Ethnopharmacol. 2014, 156, 316–346. [Google Scholar] [CrossRef] [PubMed]
- Tóth, H.B.; Blazics, B.; Kéry, Á. Polyphenol composition and antioxidant capacity of Epilobium species. J. Pharm. Biomed. Anal. 2009, 49, 26–31. [Google Scholar] [CrossRef]
- Gulçin, I.; Gören, A.C.; Taslimi, P.; Alwasel, S.H.; Kılıc, O.; Bursal, E. Anticholinergic, antidiabetic and antioxidant activities of Anatolian pennyroyal (Mentha pulegium)-analysis of its polyphenol contents by LC-MS/MS. Biocatal. Agric. Biotechnol. 2020, 23, 101441. [Google Scholar] [CrossRef]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2020, 61, 1–30. [Google Scholar] [CrossRef]
- Jääskeläinen, I.H.; Hagberg, L.; From, J.; Schyman, T.; Lehtola, L.; Järvinen, A. Treatment of complicated skin and skin structure infections in areas with low incidence of antibiotic resistance—a retrospective population based study from Finland and Sweden. Clin. Microbiol. Infect. 2016, 22, 383.e1–383.e10. [Google Scholar] [CrossRef] [Green Version]
- Bartfay, W.J.; Bartfay, E.; Johnsos, J.G. Gram-negative and gram-positive antibacterial properties of the whole plat extract of willow herb (Epilobium angustifolium). Biol. Res. Nurs. 2012, 14, 85–89. [Google Scholar] [CrossRef]
- Mukku, V.J.; Friedland, S.; Sorlie, N.E.; Donati-Lewis, H.S.; Dingmann, B.J. Antibacterial activity of selected Native American seeds. J. Med. Plant Res. 2013, 7, 2928–2932. [Google Scholar]
- Nicu, A.I.; Pîrvu, L.; Vamanu, A. Antibacterial activity of ethanolic extracts from Agrimonia eupatoria L. and Epilobium hirsutum L. herba. Sci. Bull. Ser. F Biotechnol. 2017, 21, 127–132. [Google Scholar]
- Huttunen, S.; Rihinen, K.; Kauhanen, J.; Tikkanen-Kaukanen, C. Antibacterial activity of different Finnish monofloral honeys against human pathogenic bacteria. Acta. Pathol. Microbiol. Immunol. Scand. 2013, 121, 827–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso, C.; Rubio, L.; Touriño, S.; Martí, M.; Barba, C.; Fernández-Campos, F.; Coderch, L.; Parra, J.L. Antioxidative effects and percutaneous absorption of five polyphenols. Free. Radic. Biol. Med. 2014, 75, 149–155. [Google Scholar] [CrossRef]
- Alonso, C.; Lucas, R.; Barba, C.; Martí, M.; Rubio, L.; Comelles, F.; Morales, J.C.; Coderch, L.; Parra, J.L. Skin delivery of antioxidant surfactants based on gallic acid and hydroxytyrosol. J. Pharm. Pharmacol. 2015, 67, 900–908. [Google Scholar] [CrossRef]
- Janus, E.; Ossowicz, P.; Klebeko, J.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Klimowicz, A. Enhancement of ibuprofen solubility and skin permeation by conjugation with l-valine alkyl esters. RSC Adv. 2020, 10, 7570–7584. [Google Scholar] [CrossRef] [Green Version]
- Agatonovic-Kustrin, S.; Chan, C.K.Y.; Gegechkori, V.; Morton, D.W. Models for skin and brain penetration of major components from essential oils used in aromatherapy for dementia patients. J. Biomol. Struct. Dyn. 2019, 38, 2402–2411. [Google Scholar] [CrossRef]
- Nowak, A.; Church, M.K.; Duchnik, W.; Różewicka-Czabańska, M.; Bielecka-Grzela, S.; Prowans, P.; Petriczko, J.; Czapla, N.; Bargiel, P.; Klimowicz, A. Comparison of artificial hydrophilic and lipophilic membranes and human skin to evaluate niacinamide penetration in vitro. Acta Pol. Pharm. 2020, 77, 271–279. [Google Scholar]
- Belo, S.E.D.; Gaspar, L.R.; Campos, P.M.B.G.M.; Marty, J.-P. Skin Penetration of Epigallocatechin-3-Gallate and Quercetin from Green Tea and Ginkgo biloba Extracts Vehiculated in Cosmetic Formulations. Skin Pharmacol. Physiol. 2009, 22, 299–304. [Google Scholar] [CrossRef]
- Jankowski, A.; Dyja, R.; Sarecka-Hujar, B. Dermal and Transdermal Delivery of Active Substances from Semisolid Bases. Indian J. Pharm. Sci. 2017, 79, 488–500. [Google Scholar] [CrossRef]
- Zhang, A.; Jung, E.-C.; Zhu, H.; Zou, Y.; Hui, X.; Maibach, H. Vehicle effects on human stratum corneum absorption and skin penetration. Toxicol. Ind. Health 2016, 33, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Tuntiyasawasdikul, S.; Limpongsa, E.; Jaipakdee, N.; Sripanidkulchai, B.-O. Effects of Vehicles and Enhancers on the Skin Permeation of Phytoestrogenic Diarylheptanoids from Curcuma comosa. AAPS Pharm. Sci. Tech. 2016, 18, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; White, E.T.; Howes, T.; Litster, J.D.; Marziano, I. Effect of solvent composition and temperature on the solubility of ibuprofen in aqueous ethanol. J. Chem. Eng. Data 2014, 59, 2699–2703. [Google Scholar] [CrossRef]
- Jaworska, M.; Sikora, E.; Ogonowski, J. Factors influencing the percutaneous penetration of active ingredients [Czynniki wpływające na penetrację składników aktywnych przez skórę]. Wiad. Chem. 2011, 65, 321–344. [Google Scholar]
- Bolzinger, M.-A.; Briancon, S.; Pelletier, J.; Fessi, H.; Chevalier, Y. Percutaneous release of caffeine from microemulsion, emulsion and gel dosage forms. Eur. J. Pharm. Biopharm. 2008, 68, 446–451. [Google Scholar] [CrossRef]
- Katuwavila, N.P.; Perera, A.D.L.C.; Karunaratne, V.; Amaratunga, G.; Karunaratne, D.N. Improved Delivery of Caffeic Acid through Liposomal Encapsulation. J. Nanomater 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Nitthikan, N.; Leelapornpisid, P.; Natakankitkul, S.; Chaiyana, W.; Mueller, M.; Viernstein, H.; Kiattisin, K. Improvement of Stability and Transdermal Delivery of Bioactive Compounds in Green Robusta Coffee Beans Extract Loaded Nanostructured Lipid Carriers. J. Nanotechnol. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Nowak, A.; Zielonka-Brzezicka, J.; Pechaiko, D.; Tkacz, M.; Klimowicz, A. Ocena właściwości antyoksydacyjnych liści Ginkgo biloba L. po zakończeniu wegetacji [The evaluation of the antioxidant properties of Ginkgo biloba L. leaves after the end of the growing season]. Pomeranian J. Life Sci. 2017, 63, 9–15. [Google Scholar]
- Kalisz, S.; Oszmiański, J.; Kolniak-Ostek, J.; Grobelna, A.; Kieliszek, M.; Cendrowski, A. Effect of a variety of polyphenols compounds and antioxidant properties of rhubarb (Rheum rhabarbarum). LWT 2020, 118, 108775. [Google Scholar] [CrossRef]
- Piluzza, G.; Bullitta, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm. Biol. 2010, 49, 240–247. [Google Scholar] [CrossRef]
- Tomac, I.; Šeruga, M.; Labuda, J. Evaluation of antioxidant activity of chlorogenic acids and coffee extracts by an electrochemical DNA-based biosensor. Food Chem. 2020, 325, 126787. [Google Scholar] [CrossRef] [PubMed]
- Zielonka-Brzezicka, J.; Nowak, A.; Klimowicz, A.; Wira, D.; Grzesiak, K.; Rędzikowska, E.; Wysocka, D.; Synowiec, L.; Ptak, B.; Bilska, J. Aktinidia chińska jako źródło prozdrowotnych antyoksydantów [Actinidia chinensis as a source of health-promoting antioxidants]. Probl. Hig. Epidemiol. 2018, 99, 238–244. [Google Scholar]
- Nowak, A.; Maciejewska, A.; Duchnik, W.; Florkowska, K.; Klimowicz, A. Wpływ rozpuszczalnika na właściwości antyoksydacyjne ekstraktów z zielonej herbaty (Camellia sinensis L.) [The effect of a solvent on the antioxidant properties of green tea (Camellia sinensis L.) extracts]. Probl. Hig. Epidemiol. 2018, 99, 245–258. [Google Scholar]
- Valgas, C.; de Souza, S.M.; Smania, E.F.A.; Smania, A. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar]
- Oke, M.A.; Bello, A.B.; Odebisi, M.B.; El-Imam, A.M.; Kazeem, M.O. Evaluation of antibacterial efficacy of some alcohol-based hand sanitizers sold in Ilorin (North-Central Nigeria). Ife J. Sci. 2013, 15, 111–117. [Google Scholar]
- Badran, M.; Kuntsche, J.; Fahr, A. Skin penetration enhancement by a microneedle device (Dermaroller®) in vitro: Dependency on needle size and applied formulation. Eur. J. Pharm. Sci. 2009, 36, 511–523. [Google Scholar] [CrossRef]
- Haq, A.; Michniak-Kohn, B. Effects of solvents and penetration enhancers on transdermal delivery of thymoquinone: Permeability and skin deposition study. Drug Deliv. 2018, 25, 1943–1949. [Google Scholar] [CrossRef] [Green Version]
- Kuntsche, J.; Bunjes, H.; Fahr, A.; Pappinen, S.; Rönkkö, S.; Suhonen, M.; Urtti, A. Interaction of lipid nanoparticles with human epidermis and an organotypic cell culture model. Int. J. Pharm. 2008, 354, 180–195. [Google Scholar] [CrossRef]
- Simon, A.; Amaro, M.I.; Healy, A.M.; Cabral, L.M.; De Sousa, V.P. Comparative evaluation of rivastigmine permeation from a transdermal system in the Franz cell using synthetic membranes and pig ear skin with in vivo-in vitro correlation. Int. J. Pharm. 2016, 512, 234–241. [Google Scholar] [CrossRef]
- Makuch, E.; Nowak, A.; Günther, A.; Pełech, R.; Kucharski, Ł.; Duchnik, W.; Klimowicz, A. Enhancement of the antioxidant and skin permeation properties of eugenol by the esterification of eugenol to new derivatives. AMB Express 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Kopečná, M.; Macháček, M.; Prchalová, E.; Štěpánek, P.; Drašar, P.; Kotora, M.; Vávrová, K. Galactosyl pentadecene reversibly enhances transdermal and topical drug delivery. Pharm. Res. 2017, 34, 2097–2108. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.; Ward, R.; Heylings, J. Multi-species assessment of electrical resistance as a skin integrity marker for in vitro percutaneous absorption studies. Toxicol. In Vitro 2004, 18, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.; Cristina Alonso, C.; López, O.; Rodrĭguez, G.; Coderch, L.; Notario, J.; de la Maza, A.; Parra, J.L. Barrier function of intact and impaired skin: Percutaneous penetration of caffeine and salicylic acid. Int. J. Dermatol. 2011, 50, 881–889. [Google Scholar] [CrossRef] [PubMed]









| No | Retention Time | Compound Name | Area (%) |
|---|---|---|---|
| 1 | 9.68 | Eucalyptol | 10.3 |
| 2 | 11.33 | β-Linalool | 14.8 |
| 3 | 11.57 | Camphor | 0.9 |
| 4 | 12.21 | α-Terpineol | 0.7 |
| 5 | 14.46 | α-Terpinyl acetate | 1.1 |
| 6 | 17.74 | α-Caryophyllene oxide | 1.2 |
| 7 | 18.39 | β-Caryophyllene oxide | 1.2 |
| 8 | 19.41 | 24,25-Dihydroxycholecalciferol | 7.5 |
| 9 | 20.41 | 5-Hexadecyloxy-2-pentadecyl-1,3-dioxane | 5.2 |
| 10 | 21.10 | Methyl palmitate | 15.2 |
| 11 | 22.80 | Methyl linoleate | 9.6 |
| 12 | 22.86 | Methyl oleate | 32.2 |
| Phenolic Acid Mg/Dm3 | ||||
|---|---|---|---|---|
| ChA | GA | 4-HB | 3,4-DHB | CA |
| 64.35 ± 0.53 | 241.36 ± 4.42 | 118.16 ± 4.49 | 165.19 ± 5.59 | 54.29 ± 2.25 |
| Total Polyphenols Mmol GA/Dm3 | DPPH Mmol Trolox/Dm3 | ABTS Mmol Trolox/Dm3 |
|---|---|---|
| 1.94 ± 0.06 | 3.68 ± 0.02 | 12.98 ± 0.04 |
| Strain | Extract Concentration | |||
|---|---|---|---|---|
| 100% | 50% | 25% | 12.50% | |
| Serratia lutea | 16.00 ± 0.32 a | 15.00 ± 0.06 a | 13.50 ± 0.50 ab | 8.00 ± 2.00 c |
| Serratia marcescens | 15.00 ± 0.6 a | 13.50 ± 0.05 a | 10.00 ± 0.06 b | 7.00 ± 1.00 c |
| Enterococcus faecalis | 7.00 ± 0.12 a | 6.00 ± 0.06 b | 5.00 ± 0.06 c | 5.00 ± 0.06 c |
| Enterococcus faecium | 7.00 ± 0.01 a | 6.00 ± 0.01 b | 5.00 ± 0.05 c | 5.00 ± 0.06 c |
| Streptococcus pneumoniae | 7.00 ± 0.01 a | 6.00 ± 0.06 b | 5.00 ± 0.15 c | 5.00 ± 0.06 c |
| Pseudomonas aeruginosa | 6.00 ± 0.06 a | 5.00 ± 0.06 b | 4.00 ± 0.06 c | 4.00 ± 0.010 c |
| Pseudomonas fluorescens | 6.00 ± 0.12 a | 6.00 ± 0.10 a | 6.00 ± 0.06 a | 6.00 ± 0.06 a |
| Bacillus subtilis | 11.00 ± 0,80 a | 9.50 ± 1.15 b | 7.00 ± 1.04 c | 6.50 ± 0.55 c |
| Bacillus pseudomycoides | 11.50 ± 0.58 a | 9.00 ± 1.00 b | 7.50 ± 0.50 c | 6.00 ± 0.06 c |
| Bacillus thuringiensis | 9.00 ± 0.52 a | 7.50 ± 0.58 b | 6.00 ± 0.06 c | 5.50 ± 0.50 c |
| DPPH Mmol Trolox/Dm3 | ABTS Mmol Trolox/Dm3 | Folin-Ciocalteu Mmol GA/Dm3 | |
|---|---|---|---|
| extract applied to the skin | 3.683 ± 0.048 | 12.985 ± 0.045 | 1.941 ± 0.010 |
| extract after skin extraction following 24-h penetration | 0.456 ± 0.034 | 1.622 ± 0.57 | 1.114 ± 0.106 |
| acceptor fluid after 24-h penetration | 0.216 ± 0.078 | 0.519 ± 0.107 | 0.591 ± 0.148 |
| ChA | GA | 4-HB | 3,4-DHB | CA | ||
|---|---|---|---|---|---|---|
| cumulating in the skin | µg/g skin | 110.46 ± 7.60 | 335.54 ± 51.50 | 176.18 ± 13.40 | 266.67 ± 28.43 | 119.07 ± 20.88 |
| acceptor fluid after 24 h of penetration | µg | 30.28 ± 0.97 | 80.51 ± 8.27 | 11.57 ± 3.77 | 31.93 ± 1.116 | 3.70 ± 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, A.; Cybulska, K.; Makuch, E.; Kucharski, Ł.; Różewicka-Czabańska, M.; Prowans, P.; Czapla, N.; Bargiel, P.; Petriczko, J.; Klimowicz, A. In Vitro Human Skin Penetration, Antioxidant and Antimicrobial Activity of Ethanol-Water Extract of Fireweed (Epilobium angustifolium L.). Molecules 2021, 26, 329. https://doi.org/10.3390/molecules26020329
Nowak A, Cybulska K, Makuch E, Kucharski Ł, Różewicka-Czabańska M, Prowans P, Czapla N, Bargiel P, Petriczko J, Klimowicz A. In Vitro Human Skin Penetration, Antioxidant and Antimicrobial Activity of Ethanol-Water Extract of Fireweed (Epilobium angustifolium L.). Molecules. 2021; 26(2):329. https://doi.org/10.3390/molecules26020329
Chicago/Turabian StyleNowak, Anna, Krystyna Cybulska, Edyta Makuch, Łukasz Kucharski, Monika Różewicka-Czabańska, Piotr Prowans, Norbert Czapla, Piotr Bargiel, Jan Petriczko, and Adam Klimowicz. 2021. "In Vitro Human Skin Penetration, Antioxidant and Antimicrobial Activity of Ethanol-Water Extract of Fireweed (Epilobium angustifolium L.)" Molecules 26, no. 2: 329. https://doi.org/10.3390/molecules26020329
APA StyleNowak, A., Cybulska, K., Makuch, E., Kucharski, Ł., Różewicka-Czabańska, M., Prowans, P., Czapla, N., Bargiel, P., Petriczko, J., & Klimowicz, A. (2021). In Vitro Human Skin Penetration, Antioxidant and Antimicrobial Activity of Ethanol-Water Extract of Fireweed (Epilobium angustifolium L.). Molecules, 26(2), 329. https://doi.org/10.3390/molecules26020329