Acer tataricum subsp. ginnala Inhibits Skin Photoaging via Regulating MAPK/AP-1, NF-κB, and TGFβ/Smad Signaling in UVB-Irradiated Human Dermal Fibroblasts
Abstract
:1. Introduction
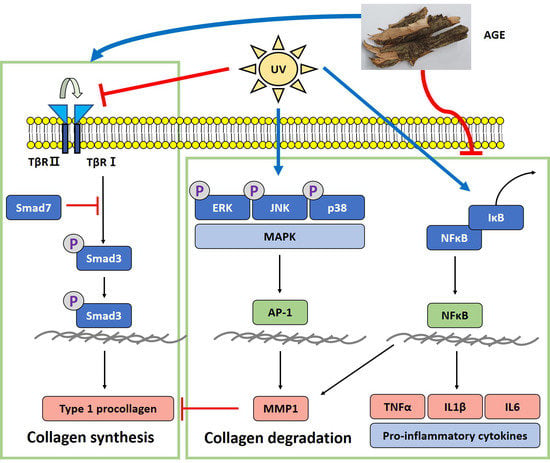
2. Results
2.1. Ultra-Performance Liquid Chromatography (UPLC) Quantitative Analysis
2.2. Cell viability after AGE Treatment in HDFs
2.3. Effect of AGE on the Expression of Type I Procollagen and MMP1 in UVB-Irradiated HDFs
2.4. Effect of AGE on MAPKs Expression in UVB-Irradiated HDFs
2.5. Effect of AGE on AP-1 Expression in UVB-Irradiated HDFs
2.6. Effect of AGE on NFκB Pathway in UVB-Irradiated HDFs
2.7. Effect of AGE on TGFβ/Smad Pathway in UVB-Irradiated HDFs
3. Discussion
4. Materials and Methods
4.1. Preparation of Acer tataricum subsp. ginnala Extract (AGE)
4.2. UPLC-PDA-ESI-MS Analysis
4.3. UPLC Quantitative Analysis
4.4. Cell Culture
4.5. UVB Irradiation and AGE Treatment
4.6. Cell Viability Assay
4.7. Determination of Type I Procollagen and MMP-1 Production
4.8. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
4.9. Western Blot Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedetto, A.V. The environment and skin aging. Clin. Dermatol. 1998, 16, 129–139. [Google Scholar] [CrossRef]
- Cavinato, M.; Jansen-Dürr, P. Molecular mechanisms of UVB-induced senescence of dermal fibroblasts and its relevance for photoaging of the human skin. Exp. Gerontol. 2017, 94, 78–82. [Google Scholar] [CrossRef]
- Tu, Y.; Quan, T. Oxidative stress and human skin connective tissue aging. Cosmetics 2016, 3, 28. [Google Scholar] [CrossRef]
- Ichihashi, M.; Ueda, M.; Budiyanto, A.; Bito, T.; Oka, M.; Fukunaga, M.; Tsuru, K.; Horikawa, T. UV-induced skin damage. Toxicology 2003, 189, 21–39. [Google Scholar] [CrossRef]
- Fisher, G.J.; Quan, T.; Purohit, T.; Shao, Y.; Cho, M.K.; He, T.; Varani, J.; Kang, S.; Voorhees, J.J. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am. J. Pathol. 2009, 174, 101–114. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxidative Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [Green Version]
- Yamaba, H.; Haba, M.; Kunita, M.; Sakaida, T.; Tanaka, H.; Yashiro, Y.; Nakata, S. Morphological change of skin fibroblasts induced by UV Irradiation is involved in photoaging. Exp. Dermatol. 2016, 25, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Munshi, A.; Ramesh, R. Mitogen-activated protein kinases and their role in radiation response. Genes Cancer 2013, 4, 401–408. [Google Scholar] [CrossRef]
- Karin, M. The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 1995, 270, 16483–16486. [Google Scholar] [CrossRef] [Green Version]
- Cooper, S.; Bowden, G. Ultraviolet B regulation of transcription factor families: Roles of nuclear factor-kappa B (NF-κB) and activator protein-1 (AP-1) in UVB-induced skin carcinogenesis. Curr. Cancer Drug Targets 2007, 7, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Karin, M. Ionizing radiation and short wavelength UV activate NF-κB through two distinct mechanisms. Proc. Natl. Acad. Sci. USA 1998, 95, 13012–13017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, T.; Quan, T.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Oxidative exposure impairs TGF-β pathway via reduction of type II receptor and SMAD3 in human skin fibroblasts. Age 2014, 36, 9623. [Google Scholar] [CrossRef] [Green Version]
- Quan, T.; He, T.; Voorhees, J.J.; Fisher, G.J. Ultraviolet irradiation blocks cellular responses to transforming growth factor-β by down-regulating its type-II receptor and inducing Smad7. J. Biol. Chem. 2001, 276, 26349–26356. [Google Scholar] [CrossRef] [Green Version]
- Smijs, T.G.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95. [Google Scholar] [CrossRef] [Green Version]
- Fruijtier-Pölloth, C. Safety assessment on polyethylene glycols (PEGs) and their derivatives as used in cosmetic products. Toxicology 2005, 214, 1–38. [Google Scholar] [CrossRef]
- Park, K.H.; Yoon, K.H.; Yin, J.; Le, T.T.; Ahn, H.S.; Yoon, S.H.; Lee, M.W. Antioxidative and anti-inflammatory activities of galloyl derivatives and antidiabetic activities of Acer ginnala. Evid.-Based Complement. Altern. Med. 2017, 2017, 6945912. [Google Scholar] [CrossRef]
- Bi, W.; He, C.-N.; Li, X.-X.; Zhou, L.-Y.; Liu, R.-J.; Zhang, S.; Li, G.-Q.; Chen, Z.-C.; Zhang, P.-F. Ginnalin A from Kujin tea (Acer tataricum subsp. ginnala) exhibits a colorectal cancer chemoprevention effect via activation of the Nrf2/HO-1 signaling pathway. Food Funct. 2018, 9, 2809–2819. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Jeong, M.-S.; Choi, S.-E.; Kim, J.-Y.; Park, K.-Y.; Park, K.-H.; Lee, D.-I.; Joo, S.-S.; Lee, C.-S.; Bang, H.-W. The effects of acer ginnala leaves extraction on the atopic dermatitis-like skin lesions in NC/Nga mice. Korean J. Dermatol. 2010, 48, 913–918. [Google Scholar]
- Bi, W.; Shen, J.; Gao, Y.; He, C.; Peng, Y.; Xiao, P. Ku-jin tea (Acer tataricum subsp. ginnala or A. tataricum subsp. theiferum), an underestimated functional beverage rich in antioxidant phenolics. J. Funct. Foods 2016, 24, 75–84. [Google Scholar] [CrossRef]
- Rittié, L.; Fisher, G.J. Natural and sun-induced aging of human skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Guo, H.; Dain, J.A.; Wan, Y.; Gao, X.-H.; Chen, H.-D.; Seeram, N.P.; Ma, H. Cytoprotective effects of a proprietary red maple leaf extract and its major polyphenol, ginnalin A, against hydrogen peroxide and methylglyoxal induced oxidative stress in human keratinocytes. Food Funct. 2020, 11, 5105–5114. [Google Scholar] [CrossRef]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Hwang, E.; Park, S.Y.; Lee, H.J.; Lee, T.Y.; Sun, Z.W.; Yi, T.H. Gallic acid regulates skin photoaging in UVB-exposed fibroblast and hairless mice. Phytother. Res. 2014, 28, 1778–1788. [Google Scholar] [CrossRef]
- Liu, C.; Xu, Y.; Kirk, R.D.; Li, H.; Li, D.; DaSilva, N.A.; Bertin, M.J.; Seeram, N.P.; Ma, H. Inhibitory effects of skin permeable glucitol-core containing gallotannins from red maple leaves on elastase and their protective effects on human keratinocytes. J. Funct. Foods 2020, 75, 104208. [Google Scholar] [CrossRef]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Cecarini, V.; Gee, J.; Fioretti, E.; Amici, M.; Angeletti, M.; Eleuteri, A.M.; Keller, J.N. Protein oxidation and cellular homeostasis: Emphasis on metabolism. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2007, 1773, 93–104. [Google Scholar] [CrossRef]
- Rosette, C.; Karin, M. Ultraviolet light and osmotic stress: Activation of the JNK cascade through multiple growth factor and cytokine receptors. Science 1996, 274, 1194–1197. [Google Scholar] [CrossRef]
- Raman, M.; Chen, W.; Cobb, M. Differential regulation and properties of MAPKs. Oncogene 2007, 26, 3100–3112. [Google Scholar] [CrossRef] [Green Version]
- Muthusamy, V.; Piva, T.J. The UV response of the skin: A review of the MAPK, NFκB and TNFα signal transduction pathways. Arch. Dermatol. Res. 2010, 302, 5. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Talwar, H.S.; Lin, J.; Lin, P.; McPhillips, F.; Wang, Z.; Li, X.; Wan, Y.; Kang, S.; Voorhees, J.J. Retinoic acid inhibits induction of c-Jun protein by ultraviolet radiation that occurs subsequent to activation of mitogen-activated protein kinase pathways in human skin in vivo. J. Clin. Investig. 1998, 101, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Liu, Z.-G.; Zandi, E. AP-1 function and regulation. Curr. Opin. Cell Biol. 1997, 9, 240–246. [Google Scholar] [CrossRef]
- Chen, Z.; Gibson, T.B.; Robinson, F.; Silvestro, L.; Pearson, G.; Xu, B.-E.; Wright, A.; Vanderbilt, C.; Cobb, M.H. MAP kinases. Chem. Rev. 2001, 101, 2449–2476. [Google Scholar] [CrossRef] [PubMed]
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- López-Camarillo, C.; Arechaga Ocampo, E.; López Casamichana, M.; Pérez-Plasencia, C.; Álvarez-Sánchez, E.; Marchat, L.A. Protein kinases and transcription factors activation in response to UV-radiation of skin: Implications for carcinogenesis. Int. J. Mol. Sci. 2012, 13, 142–172. [Google Scholar] [CrossRef]
- Ryeom, G.G.M.; Bang, W.J.; Kim, Y.B.; Lee, G.E. Gallotannin Improves the Photoaged-Related Proteins by Extracellular Signal-Regulated Kinases/c-Jun N-Terminal Kinases Signaling Pathway in Human Epidermal Keratinocyte Cells. J. Med. Food 2018, 21, 785–792. [Google Scholar] [CrossRef]
- Kondo, S. The roles of cytokines in photoaging. J. Dermatol. Sci. 2000, 23, S30–S36. [Google Scholar] [CrossRef]
- Borg, M.; Brincat, S.; Camilleri, G.; Schembri-Wismayer, P.; Brincat, M.; Calleja-Agius, J. The role of cytokines in skin aging. Climacteric 2013, 16, 514–521. [Google Scholar] [CrossRef]
- Baugé, C.; Legendre, F.; Leclercq, S.; Elissalde, J.; Pujol, J.; Galera, P.; Boumediene, K. Interleukin-1β impairment of transforming growth factor β1 signaling by DOWN-REGULATION of transforming growth factor β receptor type II and up-regulation of Smad7 in human articular chondrocytes. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2007, 56, 3020–3032. [Google Scholar] [CrossRef]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation–a review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Kook, S.-H.; Jang, Y.-S.; Lee, J.-C. Involvement of JNK-AP-1 and ERK-NF-κB signaling in tension-stimulated expression of type I collagen and MMP-1 in human periodontal ligament fibroblasts. J. Appl. Physiol. 2011, 111, 1575–1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massagué, J. TGF-β signal transduction. Annu. Rev. Biochem. 1998, 67, 753–791. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; He, T.; Kang, S.; Voorhees, J.J.; Fisher, G.J. Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-β type II receptor/Smad signaling. Am. J. Pathol. 2004, 165, 741–751. [Google Scholar] [CrossRef]
- Verrecchia, F.; Mauviel, A. Transforming growth factor-β signaling through the Smad pathway: Role in extracellular matrix gene expression and regulation. J. Investig. Dermatol. 2002, 118, 211–215. [Google Scholar] [CrossRef] [Green Version]








| Peak No. | Rt (min) | UV λ max (nm) | Formula | [M − H]− Theoretical Mass (m/z) | [M − H]− Measured Mass (m/z) | Mass Difference (mmu) | Identification |
|---|---|---|---|---|---|---|---|
| 1 | 1.514 | 220/271 | C7H6O5 | 169.013698 | 169.01369 | −0.008 | gallic acid |
| 2 | 2.892 | 215/274 | C13H16O9 | 315.071607 | 315.07041 | −1.197 | Ginnalin B/C or Maplexin A/B |
| 3 | 5.543 | 220/274 | C13H16O9 | 315.071607 | 315.07066 | −0.947 | Ginnalin B/C or Maplexin A/B |
| 4 | 6.241 | 214/277 | C20H20O13 | 467.082566 | 467.08154 | −1.026 | Maplexin C/D or 3,6-di-O-galloyl-1,5-anhydro-D-glucitol |
| 5 | 6.482 | 215/274 | C13H16O9 | 315.071607 | 315.07072 | −0.887 | Ginnalin B/C or Maplexin A/B |
| 6 | 9.200 | 215/277 | C20H20O13 | 467.082566 | 467.08605 | 3.484 | Maplexin C/D or 3,6-di-O-galloyl-1,5-anhydro-D-glucitol |
| 7 | 11.433 | 220/274 | C20H20O13 | 467.082566 | 467.08684 | 4.274 | Maplexin C/D or 3,6-di-O-galloyl-1,5-anhydro-D-glucitol |
| 8 | 13.270 | 217/275 | C20H20O13 | 467.082566 | 467.08691 | 4.344 | Maplexin C/D or 3,6-di-O-galloyl-1,5-anhydro-D-glucitol |
| 9 | 15.970 | 217/275 | C20H20O13 | 467.082566 | 467.08785 | 5.284 | Maplexin C/D or 3,6-di-O-galloyl-1,5-anhydro-D-glucitol |
| 10 | 17.764 | 224/274 | C20H20O13 | 467.082566 | 467.08779 | 5.224 | Ginnalin A (aceritannin) |
| Compound | Regression Equation | R2 | Linear Range (mg/L) | LOD (mg/L) | LOQ (mg/L) | Contents of Ginnalin A (%) |
|---|---|---|---|---|---|---|
| Ginnalin A | y = 6448.4x − 25435 | 0.9999 | 31.25–500 | 7.408 | 22.449 | 12.374 ± 0.211 |
| Gene | Sequences | |
|---|---|---|
| COL1A1 | F: AGGGCCACGAAGACATC | R: AGATGACGTCATCGCACAACA |
| MMP1 | F: CCCAAAAGCGTGTGACAGTAAG | R: CTTCCGGGTAGAAGGGATTTG |
| IL-6 | F: GTGTGAAAGCAGCAAAGAGGC | R: CTTCCGGGTAGAAGGGATTTG |
| IL1-β | F: AAAAGCTTGGTGATGTCTGG | R: TTTCAACACGCAGGACAGG |
| TNF-α | F: CAAAGTAGACCTGCCCAGAC | R: GACCTCTCTCTAATCAGCCC |
| GAPDH | F: ACCCACTCCTCCACCTTTGA | R: TGGTGGTCCAGGGGTCTTAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.-J.; Ji, Y.; Jang, Y.-P.; Choung, S.-Y. Acer tataricum subsp. ginnala Inhibits Skin Photoaging via Regulating MAPK/AP-1, NF-κB, and TGFβ/Smad Signaling in UVB-Irradiated Human Dermal Fibroblasts. Molecules 2021, 26, 662. https://doi.org/10.3390/molecules26030662
Jin Y-J, Ji Y, Jang Y-P, Choung S-Y. Acer tataricum subsp. ginnala Inhibits Skin Photoaging via Regulating MAPK/AP-1, NF-κB, and TGFβ/Smad Signaling in UVB-Irradiated Human Dermal Fibroblasts. Molecules. 2021; 26(3):662. https://doi.org/10.3390/molecules26030662
Chicago/Turabian StyleJin, Yu-Jung, Yura Ji, Young-Pyo Jang, and Se-Young Choung. 2021. "Acer tataricum subsp. ginnala Inhibits Skin Photoaging via Regulating MAPK/AP-1, NF-κB, and TGFβ/Smad Signaling in UVB-Irradiated Human Dermal Fibroblasts" Molecules 26, no. 3: 662. https://doi.org/10.3390/molecules26030662
APA StyleJin, Y. -J., Ji, Y., Jang, Y. -P., & Choung, S. -Y. (2021). Acer tataricum subsp. ginnala Inhibits Skin Photoaging via Regulating MAPK/AP-1, NF-κB, and TGFβ/Smad Signaling in UVB-Irradiated Human Dermal Fibroblasts. Molecules, 26(3), 662. https://doi.org/10.3390/molecules26030662