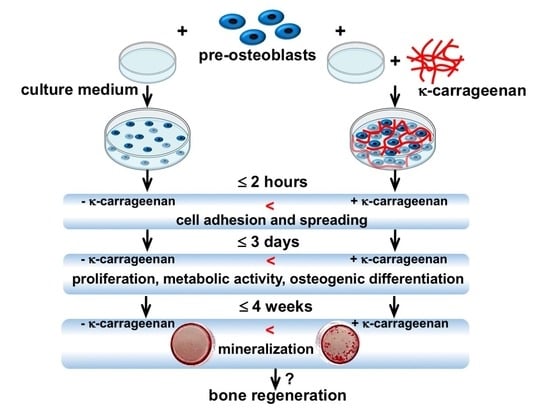
K-Carrageenan Stimulates Pre-Osteoblast Proliferation and Osteogenic Differentiation: A Potential Factor for the Promotion of Bone Regeneration?
Abstract
:1. Introduction
2. Results
2.1. Effect of K-Carrageenan on Cell Adhesion
2.2. Effect of K-Carrageenan on Cell Area and Morphology
2.3. Effect of K-Carrageenan on Paxillin Protein Expression
2.4. Effect of K-Carrageenan on Cell Migration
2.5. Effect of K-Carrageenan on Cell Metabolic Activity and Proliferation
2.6. Effect of K-Carrageenan on Osteogenic Gene Expression
2.7. Effect of K-Carrageenan on ALP Activity
2.8. Effect of K-Carrageenan on Mineralized Extracellular Matrix Production
3. Discussion
4. Materials and Methods
4.1. Preparation of K-Carrageenan
4.2. MC3T3-E1 Pre-Osteoblast Culture and Osteogenic Differentiation
4.3. Cell Adhesion
4.4. Cell Area and Morphology
4.5. Paxillin Immunofluorescence Staining
4.6. Wound Healing Scratch Assay for Cell Migration
4.7. Cell Metabolic Activity
4.8. DNA Content
4.9. Osteogenic Gene Expression
4.10. Alkaline Phosphatase (ALP) Activity
4.11. Alizarin Red Staining and Mineralized Nodule Quantification
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Van Esterik, F.A.; Zandieh-Doulabi, B.; Kleverlaan, C.J.; Klein-Nulend, J. Enhanced osteogenic and vasculogenic differentiation potential of human adipose stem cells on biphasic calcium phosphate scaffolds in fibrin gels. Stem Cells Int. 2016, 2016, 1934270. [Google Scholar] [CrossRef] [Green Version]
- Wu, V.; Helder, M.N.; Bravenboer, N.; Ten Bruggenkate, C.M.; Jin, J.; Klein-Nulend, J.; Schulten, E. Bone tissue regeneration in the oral and maxillofacial region: A review on the application of stem cells and new strategies to improve vascularization. Stem Cells Int. 2019, 2019, 6279721. [Google Scholar] [CrossRef] [Green Version]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef]
- Sun, W.; Shi, A.; Ma, D.; Bolscher, J.G.M.; Nazmi, K.; Veerman, E.C.I.; Bikker, F.J.; Lin, H.; Wu, G. All-trans retinoic acid and human salivary histatin-1 promote the spreading and osteogenic activities of pre-osteoblasts in vitro. FEBS Open Bio 2020, 10, 396–406. [Google Scholar] [CrossRef] [Green Version]
- Ansari, M. Bone tissue regeneration: Biology, strategies and interface studies. Prog. Biomater. 2019, 8, 223–237. [Google Scholar] [CrossRef] [Green Version]
- McKim, J.M., Jr.; Baas, H.; Rice, G.P.; Willoughby, J.A., Sr.; Weiner, M.L.; Blakemore, W. Effects of carrageenan on cell permeability, cytotoxicity, and cytokine gene expression in human intestinal and hepatic cell lines. Food Chem. Toxicol. 2016, 96, 1–10. [Google Scholar] [CrossRef]
- Du, L.; Brenner, T.; Xie, J.L.; Matsukawa, S. A study on phase separation behavior in kappa/iota carrageenan mixtures by micro DSC, rheological measurements and simulating water and cations migration between phases. Food Hydrocoll. 2016, 55, 81–88. [Google Scholar] [CrossRef]
- Liu, F.; Hou, P.; Zhang, H.; Tang, Q.; Xue, C.; Li, R.W. Food-grade carrageenans and their implications in health and disease. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3918–3936. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef]
- Hu, B.; Du, L.; Matsukawa, S. NMR study on the network structure of a mixed gel of kappa and iota carrageenans. Carbohydr. Polym. 2016, 150, 57–64. [Google Scholar] [CrossRef]
- Mokhtari, H.; Kharaziha, M.; Karimzadeh, F.; Tavakoli, S. An injectable mechanically robust hydrogel of kappa-carrageenan-dopamine functionalized graphene oxide for promoting cell growth. Carbohydr. Polym. 2019, 214, 234–249. [Google Scholar] [CrossRef]
- Gonzalez Ocampo, J.I.; Machado de Paula, M.M.; Bassous, N.J.; Lobo, A.O.; Ossa Orozco, C.P.; Webster, T.J. Osteoblast responses to injectable bone substitutes of kappa-carrageenan and nano hydroxyapatite. Acta Biomater. 2019, 83, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Graceffa, V.; Zeugolis, D.I. Carrageenan enhances chondrogenesis and osteogenesis in human bone marrow stem cell culture. Eur. Cell Mater. 2019, 37, 310–332. [Google Scholar] [CrossRef] [PubMed]
- Ashe, S.; Behera, S.; Dash, P.; Nayak, D.; Nayak, B. Gelatin carrageenan sericin hydrogel composites improves cell viability of cryopreserved SaOS-2 cells. Int. J. Biol. Macromol. 2020, 154, 606–620. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhan, X.; Wan, J.; Wang, Y.; Wang, C. Review for carrageenan-based pharmaceutical biomaterials: Favourable physical features versus adverse biological effects. Carbohydr. Polym. 2015, 121, 27–36. [Google Scholar] [CrossRef]
- Taghipour, Y.D.; Hokmabad, V.R.; Del Bakhshayesh, A.R.; Asadi, N.; Salehi, R.; Nasrabadi, H.T. The application of hydrogels based on natural polymers for tissue engineering. Curr. Med. Chem. 2020, 27, 2658–2680. [Google Scholar] [CrossRef] [PubMed]
- Coenen, A.M.J.; Bernaerts, K.V.; Harings, J.A.W.; Jockenhoevel, S.; Ghazanfari, S. Elastic materials for tissue engineering applications: Natural, synthetic, and hybrid polymers. Acta Biomater. 2018, 79, 60–82. [Google Scholar] [CrossRef]
- Weiner, M.L.; Ferguson, H.E.; Thorsrud, B.A.; Nelson, K.G.; Blakemore, W.R.; Zeigler, B.; Cameron, M.J.; Brant, A.; Cochrane, L.; Pellerin, M.; et al. An infant formula toxicity and toxicokinetic feeding study on carrageenan in preweaning piglets with special attention to the immune system and gastrointestinal tract. Food Chem. Toxicol. 2015, 77, 120–131. [Google Scholar] [CrossRef]
- Yoshida, M.; Yoshida, K. Sperm chemotaxis and regulation of flagellar movement by Ca2+. Mol. Hum. Reprod. 2011, 17, 457–465. [Google Scholar] [CrossRef] [Green Version]
- Castellone, R.D.; Leffler, N.R.; Dong, L.; Yang, L.V. Inhibition of tumor cell migration and metastasis by the proton-sensing GPR4 receptor. Cancer Lett. 2011, 312, 197–208. [Google Scholar] [CrossRef]
- Mishra, A.K.; Campanale, J.P.; Mondo, J.A.; Montell, D.J. Cell interactions in collective cell migration. Development 2019, 146, dev172056. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Wang, H.; Yang, J.; Cai, Y.; Hu, X.; Yang, Y.; Li, B.; Li, H.; Li, H.; Li, C.; et al. Femtosecond laser-induced micropattern and Ca/P deposition on Ti implant surface and its acceleration on early osseointegration. ACS Appl. Mater. Interfaces 2013, 5, 8179–8186. [Google Scholar] [CrossRef] [PubMed]
- Heller, M.; Kumar, V.V.; Pabst, A.; Brieger, J.; Al-Nawas, B.; Kammerer, P.W. Osseous response on linear and cyclic RGD-peptides immobilized on titanium surfaces in vitro and in vivo. J. Biomed. Mater. Res. A 2018, 106, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; O’Brien, F.J.; Little, D.G.; Schindeler, A. Cell-scaffold interactions in the bone tissue engineering triad. Eur. Cell Mater. 2013, 26, 120–132. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, J.; Chen, F.; Hou, F.; Bai, D.; Xi, P.; Zeng, Z. Biomimetic and cell-mediated mineralization of hydroxyapatite by carrageenan functionalized graphene oxide. ACS Appl. Mater. Interfaces 2014, 6, 3132–3140. [Google Scholar] [CrossRef]
- Goonoo, N.; Khanbabaee, B.; Steuber, M.; Bhaw-Luximon, A.; Jonas, U.; Pietsch, U.; Jhurry, D.; Schonherr, H. Kappa-carrageenan enhances the biomineralization and osteogenic differentiation of electrospun polyhydroxybutyrate and polyhydroxybutyrate valerate fibers. Biomacromolecules 2017, 18, 1563–1573. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Wang, F.; Chen, H.M.; Yan, X.J. Kappa-carrageenan induces the disruption of intestinal epithelial Caco-2 monolayers by promoting the interaction between intestinal epithelial cells and immune cells. Mol. Med. Rep. 2013, 8, 1635–1642. [Google Scholar] [CrossRef] [Green Version]
- Rode, M.P.; Batti Angulski, A.B.; Gomes, F.A.; da Silva, M.M.; Jeremias, T.D.S.; de Carvalho, R.G.; Iucif Vieira, D.G.; Oliveira, L.F.C.; Fernandes Maia, L.; Trentin, A.G.; et al. Carrageenan hydrogel as a scaffold for skin-derived multipotent stromal cells delivery. J. Biomater. Appl. 2018, 33, 422–434. [Google Scholar] [CrossRef]
- Overman, J.R.; Helder, M.N.; ten Bruggenkate, C.M.; Schulten, E.A.; Klein-Nulend, J.; Bakker, A.D. Growth factor gene expression profiles of bone morphogenetic protein-2-treated human adipose stem cells seeded on calcium phosphate scaffolds in vitro. Biochimie 2013, 95, 2304–2313. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Mohandas, A.; Hwang, N.S.; Jayakumar, R. Injectable angiogenic and osteogenic carrageenan nanocomposite hydrogel for bone tissue engineering. Int. J. Biol. Macromol. 2019, 122, 320–328. [Google Scholar] [CrossRef]
- Feng, W.P.; Feng, S.Y.; Tang, K.Y.; He, X.C.; Jing, A.H.; Liang, G.F. A novel composite of collagen-hydroxyapatite/kappa-carrageenan. J. Alloys Comp. 2017, 693, 482–489. [Google Scholar] [CrossRef]
- Nourmohammadi, J.; Roshanfar, F.; Farokhi, M.; Haghbin Nazarpak, M. Silk fibroin/kappa-carrageenan composite scaffolds with enhanced biomimetic mineralization for bone regeneration applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Weiner, M.L.; Nuber, D.; Blakemore, W.R.; Harriman, J.F.; Cohen, S.M. A 90-day dietary study on kappa carrageenan with emphasis on the gastrointestinal tract. Food Chem. Toxicol. 2007, 45, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; O-Sullivan, I.; Katyal, S.; Unterman, T.; Tobacman, J.K. Exposure to the common food additive carrageenan leads to glucose intolerance, insulin resistance and inhibition of insulin signalling in HepG2 cells and C57BL/6J mice. Diabetologia 2012, 55, 194–203. [Google Scholar] [CrossRef] [PubMed]








| Target Gene | Primer Sequence | |
|---|---|---|
| Runx2 | Forward Reverse | ATGCTTCATTCGCCTCAC ACTGCTTGCAGCCTTAAAT |
| Ocn | Forward Reverse | CAGACACCATGAGGACCATCTT GGTCTGATAGCTCGTCACAA |
| Fgf2 | Forward Reverse | GGCTTCTTCCTGCGCATCCA TCCGTGACCGGTAAGTATTG |
| Dmp1 | Forward Reverse | CGGCTGGTGGACTCTCTAAG CGGGGTCGTCGCTCTGCATC |
| Opn Mepe | Forward Reverse Forward Reverse | AGTGATGAAAGATGGGCAACT TCTGGACCATCTTCTTGCTGA GGAGCACTCACTACCTGAC TAGGCACTGCCACCATGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, W.; Jin, J.; Wu, G.; Bravenboer, N.; Helder, M.N.; Pathak, J.L.; Zandieh-Doulabi, B.; Hogervorst, J.M.A.; Matsukawa, S.; Geonzon, L.C.; et al. K-Carrageenan Stimulates Pre-Osteoblast Proliferation and Osteogenic Differentiation: A Potential Factor for the Promotion of Bone Regeneration? Molecules 2021, 26, 6131. https://doi.org/10.3390/molecules26206131
Cao W, Jin J, Wu G, Bravenboer N, Helder MN, Pathak JL, Zandieh-Doulabi B, Hogervorst JMA, Matsukawa S, Geonzon LC, et al. K-Carrageenan Stimulates Pre-Osteoblast Proliferation and Osteogenic Differentiation: A Potential Factor for the Promotion of Bone Regeneration? Molecules. 2021; 26(20):6131. https://doi.org/10.3390/molecules26206131
Chicago/Turabian StyleCao, Wei, Jianfeng Jin, Gang Wu, Nathalie Bravenboer, Marco N. Helder, Janak L. Pathak, Behrouz Zandieh-Doulabi, Jolanda M. A. Hogervorst, Shingo Matsukawa, Lester C. Geonzon, and et al. 2021. "K-Carrageenan Stimulates Pre-Osteoblast Proliferation and Osteogenic Differentiation: A Potential Factor for the Promotion of Bone Regeneration?" Molecules 26, no. 20: 6131. https://doi.org/10.3390/molecules26206131
APA StyleCao, W., Jin, J., Wu, G., Bravenboer, N., Helder, M. N., Pathak, J. L., Zandieh-Doulabi, B., Hogervorst, J. M. A., Matsukawa, S., Geonzon, L. C., Bacabac, R. G., Schulten, E. A. J. M., & Klein-Nulend, J. (2021). K-Carrageenan Stimulates Pre-Osteoblast Proliferation and Osteogenic Differentiation: A Potential Factor for the Promotion of Bone Regeneration? Molecules, 26(20), 6131. https://doi.org/10.3390/molecules26206131