Controlling Molecular Aggregation-Induced Emission by Controlled Polymerization
Abstract
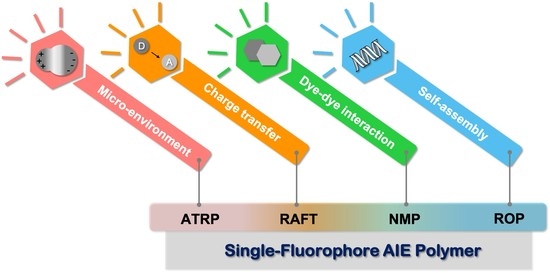
:1. Introduction
2. AIE Fluorophore-Initiated Controlled Polymerization
3. Single-Fluorophore AIE Polymers Prepared by Controlled Radical Polymerization


4. Single-Fluorophore AIE Polymers Prepared by Ring-Opening Polymerization

5. Other Well-Defined AIE Polymers
6. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Itoh, T. Fluorescence and Phosphorescence from Higher Excited States of Organic Molecules. Chem. Rev. 2012, 112, 4541–4568. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Jing, Y.N.; Li, S.S.; Su, M.; Bao, H.; Wan, W.M. Barbier Hyperbranching Polymerization-Induced Emission toward Facile Fabrication of White Light-Emitting Diode and Light-Harvesting Film. J. Am. Chem. Soc. 2019, 141, 16839–16848. [Google Scholar] [CrossRef]
- Wang, J.; Wang, N.; Wu, G.; Wang, S.; Li, X. Multicolor Emission from Non-Conjugated Polymers Based on a Single Switchable Boron Chromophore. Angew. Chem. Int. Ed. 2019, 58, 3082–3086. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Duan, Y.; Liu, B. Precise Molecular Design for High-Performance Luminogens with Aggregation-Induced Emission. Adv. Mater. 2020, 32, 1903530. [Google Scholar] [CrossRef] [Green Version]
- Hudson, Z.M.; Lunn, D.J.; Winnik, M.A.; Manners, I. Colour-tunable fluorescent multiblock micelles. Nat. Commun. 2014, 5, 3372. [Google Scholar] [CrossRef]
- Rong, Y.; Wu, C.; Yu, J.; Zhang, X.; Ye, F.; Zeigler, M.; Gallina, M.E.; Wu, I.C.; Zhang, Y.; Chan, Y.H.; et al. Multicolor Fluorescent Semiconducting Polymer Dots with Narrow Emissions and High Brightness. ACS Nano 2013, 7, 376–384. [Google Scholar] [CrossRef] [Green Version]
- Bai, K.; Wang, S.; Zhao, L.; Ding, J.; Wang, L. Efficient Blue, Green, and Red Electroluminescence from Carbazole-Functionalized Poly(spirobifluorene)s. Macromolecules 2017, 50, 6945–6953. [Google Scholar] [CrossRef]
- He, Z.; Zhao, W.; Lam, J.W.Y.; Peng, Q.; Ma, H.; Liang, G.; Shuai, Z.; Tang, B.Z. White Light Emission from a Single Organic Molecule with Dual Phosphorescence at Room Temperature. Nat. Commun. 2017, 8, 416. [Google Scholar] [CrossRef] [PubMed]
- Nagura, K.; Saito, S.; Yusa, H.; Yamawaki, H.; Fujihisa, H.; Sato, H.; Shimoikeda, Y.; Yamaguchi, S. Distinct responses to mechanical grinding and hydrostatic pressure in luminescent chromism of tetrathiazolylthiophene. J. Am. Chem. Soc. 2013, 135, 10322–10325. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Q.; Zhang, Q.-W.; Li, X.; Zhao, C.-X.; Xu, T.-Y.; Qu, D.-H.; Tian, H. Color-tunable Single-fluorophore Supramolecular System with Assembly-encoded Emission. Nat. Commun. 2020, 11, 158. [Google Scholar] [CrossRef]
- Ding, D.; Li, K.; Liu, B.; Tang, B.Z. Bioprobes Based on AIE Fluorogens. Acc. Chem. Res. 2013, 46, 2441–2453. [Google Scholar] [CrossRef] [PubMed]
- Kwok, R.T.K.; Leung, C.W.T.; Lam, J.W.Y.; Tang, B.Z. Biosensing by Luminogens with Aggregation-Induced Emission Characteristics. Chem. Soc. Rev. 2015, 44, 4228–4238. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.J.; Lam, J.W.Y.; Tang, B.Z. Tetraphenylethene: A versatile AIE building block for the construction of efficient luminescent materials for organic light-emitting diodes. J. Mater. Chem. 2012, 22, 23726–23740. [Google Scholar] [CrossRef]
- Gao, M.; Tang, B.Z. Fluorescent sensors based on aggregation-induced emission: Recent advances and perspectives. ACS Sens. 2017, 2, 1382–1399. [Google Scholar] [CrossRef]
- Yuan, Y.; Kwok, R.T.K.; Tang, B.Z.; Liu, B. Targeted Theranostic Platinum(IV) Prodrug with a Built-In Aggregation-Induced Emission Light-Up Apoptosis Sensor for Noninvasive Early Evaluation of Its Therapeutic Responses In Situ. J. Am. Chem. Soc. 2014, 136, 2546–2554. [Google Scholar] [CrossRef]
- Wang, Z.; Nie, J.; Qin, W.; Hu, Q.; Tang, B.Z. Gelation process visualized by aggregation-induced emission fluorogens. Nat. Commun. 2016, 7, 12033. [Google Scholar] [CrossRef]
- Hu, R.; Qin, A.; Tang, B.Z. AIE polymers: Synthesis and applications. Prog. Polym. Sci. 2020, 100, 101176. [Google Scholar] [CrossRef]
- Zhan, R.; Pan, Y.; Manghnani, P.N.; Liu, B. AIE Polymers: Synthesis, Properties, and Biological Applications. Macromol. Biosci. 2017, 17, 1600433. [Google Scholar] [CrossRef]
- Rodrigues, A.C.B.; de Melo, J.S.S. Aggregation-induced emission: From small molecules to polymers—historical background, mechanisms and Photophysics. Top. Curr. Chem. 2021, 15, 379. [Google Scholar]
- Tu, Y.; Zhao, Z.; Lam, J.W.Y.; Tang, B.Z. Mechanistic Connotations of Restriction of Intramolecular Motions (RIM). Natl. Sci. Rev. 2021, 8, nwaa260. [Google Scholar] [CrossRef]
- Hu, R.; Yang, X.; Qin, A.; Tang, B.Z. AIE polymers in Sensing, Imaging and Theranostic Applications. Mater. Chem. Front. 2021, 5, 4073–4088. [Google Scholar] [CrossRef]
- Hu, R.; Leung, N.L.C.; Tang, B.Z. AIE macromolecules: Syntheses, structures and functionalities. Chem. Soc. Rev. 2014, 43, 4494–4562. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Wan, H.B.; Zhou, F.; Gu, P.Y.; Xu, Q.F.; Lu, J.M. AIEgens-lightened functional polymers: Synthesis, properties and applications. Chin. J. Polym. Sci. 2019, 37, 302–326. [Google Scholar] [CrossRef]
- Corrigan, N.; Jung, K.; Moad, G.; Hawker, C.J.; Matyjaszewski, K.; Boyer, C. Reversible-deactivation radical polymerization (Controlled/living radical polymerization): From discovery to materials design and applications. Prog. Polym. Sci. 2020, 111, 101311. [Google Scholar] [CrossRef]
- Parkatzidis, K.; Wang, H.S.; Truong, N.P.; Anastasaki, A. Recent Developments and Future Challenges in Controlled Radical Polymerization: A 2020 Update. Chem 2020, 6, 1575–1588. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom Transfer Radical Polymerization (ATRP): Current Status and Future Perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Perrier, S. 50th Anniversary Perspective: RAFT Polymerization—A User Guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Nicolas, J.; Guillaneuf, Y.; Lefay, C.; Bertin, D.; Gigmes, D.; Charleux, B. Nitroxide-mediated polymerization. Prog. Polym. Sci. 2013, 38, 63–235. [Google Scholar] [CrossRef]
- Becker, G.; Wurm, F.R. Functional biodegradable polymers via ring-opening polymerization of monomers without protective groups. Chem. Soc. Rev. 2018, 47, 7739–7782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beija, M.; Charreyre, M.-T.; Martinho, J.M.G. Dye-labelled Polymer Chains at Specific Sites: Synthesis by Living/Controlled Polymerization. Prog. Polym. Sci. 2011, 36, 568–602. [Google Scholar] [CrossRef]
- Liu, S.; Rong, J.; Liu, R.; Lindsey, J.S. Single-Fluorophore Single-Chain Nanoparticle Undergoes Fluorophore-Driven Assembly with Fluorescence Features Retained in Physiological Milieu. ACS Appl. Polym. Mater. 2021, 3, 1767–1776. [Google Scholar] [CrossRef]
- Liu, R.; Lindsey, J.S. Single-Polymer–Single-Cargo Strategy Packages Hydrophobic Fluorophores in Aqueous Solution with Retention of Inherent Brightness. ACS Macro Lett. 2019, 8, 79–83. [Google Scholar] [CrossRef]
- York, A.W.; Scales, C.W.; Huang, F.; McCormick, C.L. Facile Synthetic Procedure for ω, Primary Amine Functionalization Directly in Water for Subsequent Fluorescent Labeling and Potential Bioconjugation of RAFT-Synthesized (Co)Polymers. Biomacromolecules 2007, 8, 2337–2341. [Google Scholar] [CrossRef] [PubMed]
- Bielawski, C.W.; Grubbs, R.H. Living ring-opening metathesis polymerization. Prog. Polym. Sci. 2007, 32, 1–29. [Google Scholar] [CrossRef]
- Tardy, A.; Nicolas, J.; Gigmes, D.; Lefay, C.; Guillaneuf, Y. Radical Ring-Opening Polymerization: Scope, Limitations, and Application to (Bio)Degradable Materials. Chem. Rev. 2017, 117, 1319–1406. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Liu, P.; Bai, T.; Kong, J. N-Dimethyl-Substituted boron ketoiminates for multicolor fluorescent initiators and polymers. Macromolecules 2020, 53, 3339–3348. [Google Scholar] [CrossRef]
- Mori, R.; Iasilli, G.; Lessi, M.; Muñoz-García, A.B.; Pavone, M.; Bellina, F.; Pucci, A. Luminescent solar concentrators based on PMMA films obtained from a red-emitting ATRP initiator. Polym. Chem. 2018, 9, 1168–1177. [Google Scholar] [CrossRef]
- Liu, B.; Duan, H.Y.; Wang, Y.L.; Du, B.Y.; Yang, Q.; Xu, J.T.; Yang, Y.Z.; Greiner, A.; Zhang, X.H. A fluorescein-centered polymer as a phosphor for fabricating pure white light-emitting diodes. Mater. Horiz. 2018, 5, 932–938. [Google Scholar] [CrossRef]
- Gu, P.Y.; Lu, C.J.; Ye, F.L.; Ge, J.F.; Xu, Q.F.; Hu, Z.J.; Li, N.J.; Lu, J.M. Initiator-Lightened Polymers: Preparation of End-Functionalized Polymers by ATRP and Their Intramolecular Charge Transfer and Aggregation-Induced Emission. Chem. Commun. 2012, 48, 10234–10236. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Gu, P.Y.; Zhou, J.B.; Xu, Y.J.; Liu, W.; Gu, Q.F.; Chen, D.Y.; Li, N.J.; Xu, Q.F.; Lu, J.M. Preparation of 4-Dicyanomethylene-2,6-Distyryl-4H-Pyran Derivatives, their Functional Polystyrenes and Study of their Different Aggregation Induced Emission Behaviors. J. Mater. Chem. C 2014, 2, 2082–2088. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Q.; Yang, Y.; Xing, F.; Xiao, P. PhotoATRP Approach to Poly(methyl methacrylate) with Aggregation-Induced Emission. Ind. Eng. Chem. Res. 2021, 60, 7024–7032. [Google Scholar] [CrossRef]
- Wang, X.; Qiao, X.; Yin, X.; Cui, Z.; Fu, P.; Liu, M.; Wang, G.; Pan, X.; Pang, X. Visualization of Atom Transfer Radical Polymerization by Aggregation-Induced Emission Technology. Chem. Asian J. 2020, 15, 1014–1017. [Google Scholar] [CrossRef]
- Wan, H.; Gu, P.; Zhou, F.; Wang, H.; Jiang, J.; Chen, D.; Xu, Q.; Lu, J. Polyacrylic Esters with a “One-Is-Enough” Effect and Investigation of Their AIEE Behaviors and Cyanide Detection in Aqueous Solution. Polym. Chem. 2018, 9, 3893–3899. [Google Scholar] [CrossRef]
- Gu, P.Y.; Zhang, Y.H.; Chen, D.Y.; Lu, C.J.; Zhou, F.; Xu, Q.F.; Lu, J.M. Tuning the fluorescence of aggregates for end-functionalized polymers through varying polymer chains with different polarities. RSC Adv. 2015, 5, 8167–8174. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, S.; Song, F.; Khorloo, M.; Zhang, H.; Kwok, R.T.K.; Lam, J.W.Y.; He, Z.; Tang, B.Z. Facile Emission Color Tuning and Circularly Polarized Light Generation of Single Luminogen in Engineering Robust Forms. Mater. Horiz. 2019, 6, 405–411. [Google Scholar] [CrossRef]
- Ye, S.; Tian, T.; Christofferson, A.J.; Erikson, S.; Jagielski, J.; Luo, Z.; Kumar, S.; Shih, C.-J.; Leroux, J.-C.; Bao, Y. Continuous color tuning of single-fluorophore emission via polymerization-mediated through-space charge transfer. Sci. Adv. 2021, 7, eabd1794. [Google Scholar] [CrossRef]
- Hu, J.; Li, Q.; Wang, X.; Shao, S.; Wang, L.; Jing, X.; Wang, F. Developing Through-Space Charge Transfer Polymers as a General Approach to Realize Full-Color and White Emission with Thermally Activated Delayed Fluorescence. Angew. Chem. Int. Ed. 2019, 58, 8405–8409. [Google Scholar] [CrossRef] [PubMed]
- Tonge, C.M.; Hudson, Z.M. Interface-Dependent Aggregation-Induced Delayed Fluorescence in Bottlebrush Polymer Nanofibers. J. Am. Chem. Soc. 2019, 141, 13970–13976. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhang, X.; Zhang, X.; Fu, C.; Wang, K.; Yuan, J.; Tao, L.; Wei, Y. Amphiphilic fluorescent copolymers via one-pot combination of chemoenzymatic transesterification and RAFT polymerization: Synthesis, self-assembly and cell imaging. Polym. Chem. 2015, 6, 607–612. [Google Scholar] [CrossRef]
- Zeng, G.; Liu, M.; Jiang, R.; Huang, Q.; Huang, L.; Wan, Q.; Dai, Y.; Wen, Y.; Zhang, X.; Wei, Y. Self-catalyzed Photo-initiated RAFT Polymerization for Fabrication of Fluorescent Polymeric Nanoparticles with Aggregation-induced Emission Feature. Mater. Sci. Eng. C 2018, 83, 154–159. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Y.; Chen, S.; Deng, H.; Zhu, X. Building single-color AIE-active reversible micelles to interpret temperature and pH stimuli in both solutions and cells. Macromolecules 2018, 51, 5234–5244. [Google Scholar] [CrossRef]
- Zhou, M.; Li, L.; Xie, W.; He, Z.; Li, J. Synthesis of a Thermal-Responsive Dual-Modal Supramolecular Probe for Magnetic Resonance Imaging and Fluorescence Imaging. Macromol. Rapid Commun. 2021, 42, 2100248. [Google Scholar] [CrossRef]
- Chen, Y.J.; Han, H.J.; Tong, H.X.; Chen, T.T.; Wang, H.B.; Ji, J.; Jin, Q. Zwitterionic Phosphorylcholine-TPE Conjugate for pH-Responsive Drug Delivery and AIE Active Imaging. ACS Appl. Mater. Interfaces 2016, 8, 21185–21192. [Google Scholar] [CrossRef]
- Yu, T.; Zhuang, W.; Su, X.; Ma, B.; Hu, J.; He, H.; Li, G.; Wang, Y. Dual-responsive micelles with aggregation-induced emission feature and two-photon aborsption for accurate drug delivery and bioimaging. Bioconjugate Chem. 2019, 30, 2075–2087. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Wang, Y.; Li, L.; Zhou, N.; Cai, Y.; Zhang, Z.; Zhu, X. Azoreductase-triggered fluorescent nanoprobe synthesized by RAFT-mediated polymerization-induced self-assembly for drug release. Polym. Chem. 2020, 11, 5619–5629. [Google Scholar] [CrossRef]
- Liu, S.; Cheng, Y.; Zhang, H.; Qiu, Z.; Kwok, R.T.; Lam, J.W.; Tang, B.Z. In Situ Monitoring of RAFT Polymerization by Tetraphenylethylene-Containing Agents with Aggregation-Induced Emission Characteristics. Angew. Chem. Int. Ed. 2018, 57, 6274–6278. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Wu, Z.Y.; Sun, Y.H.; Fang, J.L.; Chen, D.Z. Highly Efficient Luminescent Side-Chain Polymers with Short-Spacer Attached TetraphenylethyleneAIEgens via RAFT Polymerization Capable of Naked Eye Explosive Detection. Polym. Chem. 2018, 9, 4150–4160. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, L.; Zhang, P.; Zhao, E.; Zhou, L.; Chen, D.; Sun, J.; Gu, X.; Yang, W.; Tang, B.Z. Fluorescence Self-Reporting Precipitation Polymerization Based on Aggregation-Induced Emission for Constructing Optical Nanoagents. Angew. Chem. Int. Ed. 2020, 59, 10122–10128. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Guégain, E.; Nicolas, V.; Nicolas, J. Fluorescent Polymer Prodrug Nanoparticles with Aggregation-Induced Emission (AIE) Properties from Nitroxide-Mediated Polymerization. Chem. Commun. 2017, 53, 4489–4492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, Y.; Boissenot, T.; Guégain, E.; Desmaële, D.; Mura, S.; Couvreur, P.; Nicolas, J. Simple Synthesis of Cladribine-Based Anticancer Polymer Prodrug Nanoparticles with Tunable Drug Delivery Properties. Chem. Mater. 2016, 28, 6266–6275. [Google Scholar] [CrossRef]
- Bao, Y.; Nicolas, J. Structure–cytotoxicity relationship of drug-initiated polymer prodrug nanoparticles. Polym. Chem. 2017, 8, 5174–5184. [Google Scholar] [CrossRef]
- Bao, Y.; Guégain, E.; Mougin, J.; Nicolas, J. Self-stabilized, hydrophobic or PEGylated paclitaxel polymer prodrug nanoparticles for cancer therapy. Polym. Chem. 2018, 9, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Yong, T.-Y.; Wan, J.; Li, Z.-H.; Zhao, H.; Zhao, Y.; Gan, L.; Yang, X.-L.; Xu, H.-B.; Zhang, C. Temperature-Sensitive Fluorescent Organic Nanoparticles with Aggregation-Induced Emission for Long-Term Cellular Tracing. ACS Appl. Mater. Interfaces 2015, 7, 3420–3425. [Google Scholar] [CrossRef]
- Feng, W.; Li, G.; Tao, L.; Wei, Y.; Wang, X. Poly(amino acid)s-based star AIEgens for cell uptake with pH-response and chiral difference. Colloids Surf. B Biointerfaces 2021, 202, 111687. [Google Scholar] [CrossRef] [PubMed]
- Kamber, N.E.; Jeong, W.; Waymouth, R.M. Organocatalytic Ring-Opening Polymerization. Chem. Rev. 2007, 107, 5813–5840. [Google Scholar] [CrossRef]
- Huang, J.; Heise, A. Stimuli responsive synthetic polypeptides derived from N-carboxyanhydride (NCA) polymerisation. Chem. Soc. Rev. 2013, 42, 7373–7390. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Tan, Z.; Cheng, J. Recent Advances and Future Perspectives of Synthetic Polypeptides from N-Carboxyanhydrides. Macromolecules 2019, 52, 8521–8539. [Google Scholar] [CrossRef]
- Zhang, G.; Palmer, G.M.; Dewhirst, M.W.; Fraser, C.L. A Dual-Emissive-Materials Design Concept Enables Tumour Hypoxia Imaging. Nat. Mater. 2009, 8, 747–751. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Chen, J.; Payne, S.J.; Kooi, S.E.; Demas, J.N.; Fraser, C.L. Multi-emissive difluoroboron dibenzoylmethane polylactide exhibiting intense fluorescence and oxygen-sensitive room-temperature phosphorescence. J. Am. Chem. Soc. 2007, 129, 8942–8943. [Google Scholar] [CrossRef]
- Bao, Y.; De Keersmaecker, H.; Corneillie, S.; Yu, F.; Mizuno, H.; Zhang, G.; Hofkens, J.; Mendrek, B.; Kowalczuk, A.; Smet, M. Tunable Ratiometric Fluorescence Sensing of Intracellular pH by Aggregation-Induced Emission-Active Hyperbranched Polymer Nanoparticles. Chem. Mater. 2015, 27, 3450–3455. [Google Scholar] [CrossRef]
- Li, S.T.; Lin, Y.C.; Kuo, S.W.; Chuang, W.T.; Hong, J.L. Aggregation Induced Emission Enhancement in Relation to the Secondary Structures of Poly (γ-Benzyl-l-Glutamate) Containing a Fluorescent Tetraphenylthiophene Moiety. Polym. Chem. 2012, 3, 2393–2402. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Huang, P.-C.; Yang, D.-J.; Gao, J.-Y.; Hong, J.-L. Influence of the secondary structure on the AIE-related emission behavior of an amphiphilic polypeptide containing a hydrophobic fluorescent terminal and hydrophilic pendant groups. Polym. Chem. 2016, 7, 153–163. [Google Scholar] [CrossRef]
- Shih, K.Y.; Hsiao, T.S.; Deng, S.L.; Hong, J.L. Water-Soluble Poly (γ-propargyl-L-glutamate) Containing Pendant Sulfonate Ions and Terminal Fluorophore: Aggregation-Enhanced Emission and Secondary Structure. Macromolecules 2014, 47, 4037–4047. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Lu, F.-H.; Hong, J.-L.; Kuo, S.-W. Strong emission of 2,4,6-triphenylpyridine-functionalized polytyrosine and hydrogen-bonding interactions with poly (4-vinylpyridine). Polym. Chem. 2015, 6, 6340–6350. [Google Scholar] [CrossRef]
- Liang, G.D.; Ren, F.; Gao, H.Y.; Wu, Q.; Zhu, F.M.; Tang, B.Z. Continuously-Tunable Fluorescent Polypeptides through a Polymer-Assisted Assembly Strategy. Polym. Chem. 2016, 7, 5181–5187. [Google Scholar] [CrossRef]
- El-Mahdy, A.F.M.; Kuo, S.-W. A pyrene-functionalized polytyrosine exhibiting aggregation-induced emission and capable of dispersing carbon nanotubes and hydrogen bonding with P4VP. Polymer 2018, 156, 10–21. [Google Scholar] [CrossRef]
- Tian, J.; Jiang, R.; Gao, P.; Xu, D.; Mao, L.; Zeng, G.; Liu, M.; Deng, F.; Zhang, X.; Wei, Y. Synthesis and cell imaging applications of amphiphilic AIE-active poly(amino acid)s. Mater. Sci. Eng. C 2017, 79, 563–569. [Google Scholar] [CrossRef]
- Jérôme, C.; Lecomte, P. Recent advances in the synthesis of aliphatic polyesters by ring-opening polymerization. Adv. Drug Delivery Rev. 2008, 60, 1056–1076. [Google Scholar] [CrossRef]
- Paunović, N.; Bao, Y.; Coulter, F.B.; Masania, K.; Geks, A.K.; Klein, K.; Rafsanjani, A.; Cadalbert, J.; Kronen, P.W.; Kleger, N.; et al. Digital Light 3D Printing of Customized Bioresorbable Airway Stents with Elastomeric Properties. Sci. Adv. 2021, 7, abe9499. [Google Scholar] [CrossRef] [PubMed]
- Sandmeier, M.; Paunović, N.; Conti, R.; Hofmann, L.; Wang, J.; Luo, Z.; Masania, K.; Wu, N.; Kleger, N.; Coulter, F.B.; et al. Solvent-Free Three-Dimensional Printing of Biodegradable Elastomers Using Liquid Macrophotoinitiators. Macromolecules 2021, 54, 7830–7839. [Google Scholar] [CrossRef]
- Jia, W.; Yang, P.; Li, J.; Yin, Z.; Kong, L.; Lu, H.; Ge, Z.; Wu, Y.; Hao, X.; Yang, J. Synthesis and Characterization of a Novel Cyanostilbene Derivative and Its Initiated Polymers: Aggregation-Induced Emission Enhancement Behaviors and Light-Emitting Diode Applications. Polym. Chem. 2014, 5, 2282–2292. [Google Scholar] [CrossRef]
- Zhang, G.; Kooi, S.E.; Demas, J.N.; Fraser, C.L. Emission Color Tuning with Polymer Molecular Weight for Difluoroboron Dibenzoylmethane-Polylactide. Adv. Mater. 2008, 20, 2099–2104. [Google Scholar] [CrossRef]
- Zhang, G.; St. Clair, T.L.; Fraser, C.L. Synthesis and Fluorescent Properties of Difluoroboron Dibenzoylmethane Polycaprolactone. Macromolecules 2009, 42, 3092–3097. [Google Scholar] [CrossRef]
- Wang, K.; Luo, Y.; Huang, S.; Yang, H.; Liu, B.; Wang, M. Highly fluorescent polycaprolactones decorated with di(thiophene-2-yl)-diketopyrrolopyrrole: A covalent strategy of tuning fluorescence properties in solid states. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1032–1042. [Google Scholar] [CrossRef]
- Zhao, W.; Li, C.; Liu, B.; Wang, X.; Li, P.; Wang, Y.; Wu, C.; Yao, C.; Tang, T.; Liu, X. A New Strategy to Access Polymers with Aggregation-Induced Emission Characteristics. Macromolecules 2014, 47, 5586–5594. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.; He, S.; Huang, Y.; Cui, D. Synthesis and AIE Properties of PEG–PLA–PMPC Based Triblock Amphiphilic Biodegradable Polymers. Polym. Chem. 2016, 7, 1121–1128. [Google Scholar] [CrossRef]
- Wang, E.; Liu, S.; Lam, J.W.Y.; Tang, B.Z.; Wang, X.; Wang, F. Deciphering Structure–Functionality Relationship of Polycarbonate-Based Polyelectrolytes by AIE Technology. Macromolecules 2020, 53, 5839–5846. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, H.; Fan, Y.; Zhou, L.; Trépout, S.; Guo, J.; Li, M.-H. Fluorescent Polymersomes with Aggregation-Induced Emission. ACS Nano 2018, 12, 4025–4035. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Ye, Q.; Che, H.; Wang, X.; Wei, Y.; Yuan, J. Polymer Assemblies with Nanostructure-Correlated Aggregation-Induced Emission. Macromolecules 2017, 50, 1126–1133. [Google Scholar] [CrossRef]
- Nagarajan, S.; Sankar, V.; Bejoymohandas, K.S.; Duan, Y.; Zhang, J. Influence of Branched Polyester Chains on the Emission Behavior of Dipyridamole Molecule and Its Biosensing Ability. ACS Omega 2018, 3, 15530–15537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, Z.; Lang, M. Controllable synthesis and AIE properties of fluorescent polyesters. Eur. Polym. J. 2018, 109, 297–302. [Google Scholar] [CrossRef]
- Dong, J.; Jiang, R.; Huang, H.; Chen, J.; Tian, J.; Deng, F.; Dai, Y.; Wen, Y.; Zhang, X.; Wie, Y. Facile preparation of fluorescent nanodiamond based polymer nanoparticles via ring-opening polymerization and their biological imaging. Mater. Sci. Eng. C 2020, 106, 110297. [Google Scholar] [CrossRef]
- Yang, R.; Wang, Y.; Luo, W.; Jin, Y.; Zhang, Z.; Wu, C.; Hadjichristidis, N. Carboxylic Acid Initiated Organocatalytic Ring-Opening Polymerization of N-Sulfonyl Aziridines: An Easy Access to Well-Controlled Polyaziridine-Based Architectural and Functionalized Polymers. Macromolecules 2019, 52, 8793–8802. [Google Scholar] [CrossRef]
- Jiang, Y.; Hadjichristidis, N. Tetraphenylethene-Functionalized Polyethylene-Based Polymers with Aggregation-Induced Emission. Macromolecules 2019, 52, 1955–1964. [Google Scholar] [CrossRef]
- Luo, J.; Shea, K.J. Polyhomologation. A Living C1 Polymerization. Acc. Chem. Res. 2010, 43, 1420–1433. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, X.; Yang, B.; Deng, F.; Yang, Y.; Li, Z.; Zhang, X.; Wei, Y. Preparation and bioimaging applications of AIE dye cross-linked luminescent polymeric nanoparticles. Macromol. Biosci. 2014, 14, 1712–1718. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, X.; Wang, K.; Zhang, X.; Zhou, Y.; Liu, H.; Wei, Y. A biocompatible cross-linked fluorescent polymer prepared via ring-opening PEGylation of 4-arm PEG-amine, itaconic anhydride, and an AIE monomer. Polym. Chem. 2015, 6, 3634–3640. [Google Scholar] [CrossRef]
- Pesenti, T.; Nicolas, J. 100th Anniversary of Macromolecular Science Viewpoint: Degradable Polymers from Radical Ring-Opening Polymerization: Latest Advances, New Directions, and Ongoing Challenges. ACS Macro Lett. 2020, 9, 1812–1835. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Y.; Yan, G.; Zhang, K. Aggregation-Induced Emission Block Copolymers Based on Ring-Opening Metathesis Polymerization. RSC Adv. 2014, 4, 51194–51200. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, W.; Ren, L.; Zhang, K. Aggregation-Induced Emission Polymer Nanoparticles with pH-Responsive Fluorescence. Polym. Chem. 2016, 7, 5386–5395. [Google Scholar] [CrossRef]
- Parke, S.M.; Hupf, E.; Matharu, G.K.; de Aguiar, I.; Xu, L.; Yu, H.; Boone, M.P.; de Souza, G.L.C.; McDonald, R.; Ferguson, M.J.; et al. Aerobic Solid State Red Phosphorescence from Benzobismole Monomers and Patternable Self-Assembled Block Copolymers. Angew. Chem. Int. Ed. 2018, 57, 14841–14846. [Google Scholar] [CrossRef]
- Wu, Y.; Qu, L.; Li, J.; Huang, L.; Liu, Z. A versatile method for preparing well-defined polymers with aggregation-induced emission property. Polymer 2018, 158, 297–307. [Google Scholar] [CrossRef]
- Cappello, D.; Watson, A.E.R.; Gilroy, J.B. A Boron Difluoride Hydrazone (BODIHY) Polymer Exhibits Aggregation-Induced Emission. Macromol. Rapid Commun. 2021, 42, 2000553. [Google Scholar] [CrossRef]
- Chen, C.-H.; Satyanarayana, K.; Liu, Y.-H.; Huang, S.-L.; Lim, T.-S.; Luh, T.-Y. Excimer formation in a confined space. photophysics of ladderphanes with tetraarylethylene linkers. Chem. Eur. J. 2015, 21, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Madkour, A.E.; Koch, A.H.R.; Lienkamp, K.; Tew, G.N. End-Functionalized ROMP Polymers for Biomedical Applications. Macromolecules 2010, 43, 4557–4561. [Google Scholar] [CrossRef] [Green Version]
- Roberts, K.S.; Sampson, N.S. A Facile Synthetic Method to Prepare Fluorescently Labeled ROMP Polymers. Org. Lett. 2004, 6, 3253–3255. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; McGonigal, P.R.; Ye, R.; Liu, S.; Lam, J.W.Y.; Kwok, R.T.K.; Yuan, W.Z.; Xie, J.; Rogach, A.L.; et al. Clusterization-triggered emission: Uncommon luminescence from common materials. Mater. Today 2020, 32, 275–292. [Google Scholar] [CrossRef]
- Cao, S.; Xia, Y.; Shao, J.; Guo, B.; Dong, Y.; Pijpers, I.A.B.; Zhong, Z.; Meng, F.; Abdelmohsen, L.K.E.A.; Williams, D.S.; et al. Biodegradable Polymersomes with Structure Inherent Fluorescence and Targeting Capacity for Enhanced Photo-Dynamic Therapy. Angew. Chem. Int. Ed. 2021, 60, 17629–17637. [Google Scholar] [CrossRef] [PubMed]
- Paisley, N.R.; Halldorson, S.V.; Tran, M.V.; Gupta, R.; Kamal, S.; Algar, W.R.; Hudson, Z.M. Near-Infrared-Emitting Boron-Difluoride-Curcuminoid-Based Polymers Exhibiting Thermally Activated Delayed Fluorescence as Biological Imaging Probes. Angew. Chem. Int. Ed. 2021, 60, 18630–18638. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Shao, J.; Wu, H.; Song, S.; De Martino, M.T.; Pijpers, I.A.; Friedrich, H.; Abdelmohsen, L.K.; Williams, D.S.; van Hest, J.C. Photoactivated Nanomotors Via Aggregation Induced Emission for Enhanced Phototherapy. Nat. Comm. 2021, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.J.; Li, Y.; Xu, J.C.; Lu, H.F.; Li, Y.; Song, D.P. Self-Assembled Photonic Microsensors with Strong Aggregation-Induced Emission for Ultra-Trace Quantitative Detection. ACS Nano 2021, 15, 5534–5544. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Z.; Zhao, X.; Yu, F.; Quan, Y.; Cheng, Y.; Yuan, H. DOX Loaded Aggregation-induced Emission Active Polymeric Nanoparticles as a Fluorescence Resonance Energy Transfer Traceable Drug Delivery System for Self-indicating Cancer Therapy. Acta Biomater. 2019, 85, 218–228. [Google Scholar] [CrossRef]
- Wang, Y.B.; Shi, L.L.; Ma, D.; Xu, S.D.; Wu, W.B.; Xu, L.; Panahandeh-Fard, M.; Zhu, X.Y.; Wang, B.; Liu, B. Tumor-Activated and Metal-Organic Framework Assisted Self-Assembly of Organic Photosensitizers. ACS Nano 2020, 14, 13056–13068. [Google Scholar] [CrossRef]
- Ju, C.-W.; Bai, H.; Li, B.; Liu, R. Machine learning enables highly accurate predictions of photophysical properties of organic fluorescent materials: Emission wavelengths and quantum yields. J. Chem. Inf. Model. 2021, 61, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Westermayr, J.; Marquetand, P. Machine Learning for Electronically Excited States of Molecules. Chem. Rev. 2021, 121, 9873–9926. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, Y. Controlling Molecular Aggregation-Induced Emission by Controlled Polymerization. Molecules 2021, 26, 6267. https://doi.org/10.3390/molecules26206267
Bao Y. Controlling Molecular Aggregation-Induced Emission by Controlled Polymerization. Molecules. 2021; 26(20):6267. https://doi.org/10.3390/molecules26206267
Chicago/Turabian StyleBao, Yinyin. 2021. "Controlling Molecular Aggregation-Induced Emission by Controlled Polymerization" Molecules 26, no. 20: 6267. https://doi.org/10.3390/molecules26206267
APA StyleBao, Y. (2021). Controlling Molecular Aggregation-Induced Emission by Controlled Polymerization. Molecules, 26(20), 6267. https://doi.org/10.3390/molecules26206267