A New Dimeric Copper(II) Complex of Hexyl Bis(pyrazolyl)acetate Ligand as an Efficient Catalyst for Allylic Oxidations
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
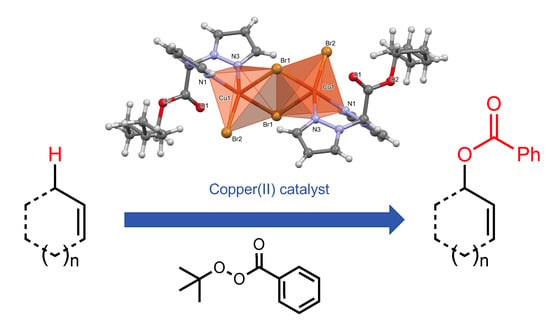
2.2. Investigation on the Catalytic Activity of Complex 1 in the Allylic Oxidation of Alkenes
2.3. X-ray Crystallography
3. Experimental Section
3.1. Materials and Instruments
3.2. Synthesis
3.2.1. Synthesis of HC(COOHex)(pz)2 (LOHex)
3.2.2. Synthesis of [Cu(LOHex)Br(μ-Br)]2 (1)
3.2.3. General Procedure for the Synthesis of Compounds 4–6
Synthesis of 4
Synthesis of 5
Synthesis of 6
3.3. Crystallographic Data Collection and Refinement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- White, M.C. C–H Bond Functionalization & Synthesis in the 21st Century: A Brief History and Prospectus. Synlett 2012, 23, 2746–2748. [Google Scholar]
- Wencel-Delord, J.; Dröge, T.; Liu, F.; Glorius, F. Towards mild metal-catalyzed C–H bond activation. Chem. Soc. Rev. 2011, 40, 4740–4761. [Google Scholar] [CrossRef]
- Newhouse, T.; Baran, P.S. If C–H Bonds Could Talk: Selective C-H Bond Oxidation. Angew. Chem. Int. Ed. Engl. 2011, 50, 3362–3374. [Google Scholar] [CrossRef] [Green Version]
- Bergman, R.G. C–H activation. Nature 2007, 446, 391–393. [Google Scholar] [CrossRef]
- Bregeault, J.M. Transition-metal complexes for liquid-phase catalytic oxidation: Some aspects of industrial reactions and of emerging technologies. Dalton Trans. 2003, 17, 3289–3302. [Google Scholar] [CrossRef]
- Ullmann, F.; Wolfgang, G.; Yamamoto, Y.S.; Campbell, F.T.; Pfefferkorn, R.; Rounsaville, J. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany; Deerfield Beach, FL, USA, 1985; Volume A3. [Google Scholar]
- Rawlinson, D.J.; Sosnovsky, G. One-Step Substitutive Acyloxylation at Carbon. Part I. Reactions Involving Peroxides. Synthesis 1972, 1972, 1–28. [Google Scholar] [CrossRef]
- García-Cabeza, A.L.; Moreno-Dorado, F.J.; Ortega, M.J.; Guerra, F.M. Copper-Catalyzed Oxidation of Alkenes and Heterocycles. Synthesis 2016, 48, 2323–2342. [Google Scholar]
- Zhu, N.; Qian, B.; Xiong, H.; Bao, H. Copper-catalyzed regioselective allylic oxidation of olefins via C–H activation. Tetrahedron Lett. 2017, 58, 4125–4128. [Google Scholar] [CrossRef]
- Andrus, M.B.; Lashley, J.C. Copper catalyzed allylic oxidation with peresters. Tetrahedron 2002, 58, 845–866. [Google Scholar] [CrossRef]
- Kharasch, M.S.; Sosnovsky, G. The Reactions of t-Butyl Perbenzoate and Olefins—A Stereospecific Reaction. J. Am. Chem. Soc. 1958, 80, 756. [Google Scholar] [CrossRef]
- Kharasch, M.; Fono, A. Communications-A New Method of Introducing Peroxy Groups into Organic Molecules. J. Org. Chem. 1958, 23, 324–325. [Google Scholar] [CrossRef]
- Kharasch, M.; Sosnovsky, G.; Yang, N. Reactions of t-butyl Peresters. I. The reaction of Peresters with olefins1. J. Am. Chem. Soc. 1959, 81, 5819–5824. [Google Scholar] [CrossRef]
- Fraunhoffer, K.J. 7.05 Oxidation Adjacent to CC Bonds. In Comprehensive Organic Synthesis, 2nd ed.; Knochel, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 145–177. [Google Scholar]
- Weidmann, V.; Maison, W. Allylic Oxidations of Olefins to Enones. Synthesis 2013, 45, 2201–2221. [Google Scholar]
- McLaughlin, E.C.; Choi, H.; Wang, K.; Chiou, G.; Doyle, M.P. Allylic Oxidations Catalyzed by Dirhodium Caprolactamate via Aqueous tert-Butyl Hydroperoxide: The Role of the tert-Butylperoxy Radical. J. Org. Chem. 2009, 74, 730–738. [Google Scholar] [CrossRef] [Green Version]
- Delcamp, J.H.; White, M.C. Sequential Hydrocarbon Functionalization: Allylic C–H Oxidation/Vinylic C–H Arylation. J. Am. Chem. Soc. 2006, 128, 15076–15077. [Google Scholar] [CrossRef]
- Chen, M.S.; White, M.C. A Sulfoxide-Promoted, Catalytic Method for the Regioselective Synthesis of Allylic Acetates from Monosubstituted Olefins via C–H Oxidation. J. Am. Chem. Soc. 2004, 126, 1346–1347. [Google Scholar] [CrossRef]
- Catino, A.J.; Forslund, R.E.; Doyle, M.P. Dirhodium(II) Caprolactamate: An Exceptional Catalyst for Allylic Oxidation. J. Am. Chem. Soc. 2004, 126, 13622–13623. [Google Scholar] [CrossRef] [PubMed]
- Eames, J.; Watkinson, M. Catalytic allylic oxidation of alkenes using an asymmetric Kharasch-Sosnovsky reaction. Angew. Chem. Int. Ed. Engl. 2001, 40, 3567–3571. [Google Scholar] [CrossRef]
- Brunel, J.M.; Legrand, O.; Buono, G. Recent advances in asymmetric copper allylic oxidation of olefins. Comptes Rendus Acad. Sci. Ser. II C 1999, 2, 19–23. [Google Scholar] [CrossRef]
- Chemler, S.R. Copper catalysis in organic synthesis. Beilstein J. Org. Chem. 2015, 11, 2252–2253. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, A.; Nakada, M. Allylic Oxidations in Natural Product Synthesis. Synthesis 2013, 45, 1421–1451. [Google Scholar]
- Tan, Q.; Hayashi, M. Asymmetric Desymmetrization of 4,5-Epoxycyclohex-1-ene by Enantioselective Allylic Oxidation. Org. Lett. 2009, 11, 3314–3317. [Google Scholar] [CrossRef]
- Tanaka, T.; Tan, Q.; Kawakubo, H.; Hayashi, M. Formal Total Synthesis of (−)-Oseltamivir Phosphate. J. Org. Chem. 2011, 76, 5477–5479. [Google Scholar] [CrossRef]
- Badreddine, A.; Karym, E.M.; Zarrouk, A.; Nury, T.; El Kharrassi, Y.; Nasser, B.; Cherkaoui Malki, M.; Lizard, G.; Samadi, M. An expeditious synthesis of spinasterol and schottenol, two phytosterols present in argan oil and in cactus pear seed oil, and evaluation of their biological activities on cells of the central nervous system. Steroids 2015, 99, 119–124. [Google Scholar] [CrossRef]
- Rahman, F.U.; Rahman, A.U.; Tan, T.W. Direct Cu-Catalyzed Allylic Acetoxylation of Δ5-Steroids at 7-Position. J. Chin. Chem. Soc. 2010, 57, 1237–1242. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, W.; Sui, Z. Concise Enantioselective Syntheses of Quinolactacins A and B through Alternative Winterfeldt Oxidation. J. Org. Chem. 2003, 68, 4523–4526. [Google Scholar] [CrossRef]
- Patin, A.; Kanazawa, A.; Philouze, C.; Greene, A.E.; Muri, E.; Barreiro, E.; Costa, P.C.C. Highly Stereocontrolled Synthesis of Natural Barbacenic Acid, Novel Bisnorditerpene from Barbacenia flava. J. Org. Chem. 2003, 68, 3831–3837. [Google Scholar] [CrossRef] [PubMed]
- Ginotra, S.K.; Singh, V.K. Enantioselective oxidation of olefins catalyzed by chiral copper bis(oxazolinyl)pyridine complexes: A reassessment. Tetrahedron 2006, 62, 3573–3581. [Google Scholar] [CrossRef]
- Aldea, L.; Garcia, J.I.; Mayoral, J.A. Multiphase enantioselective Kharasch-Sosnovsky allylic oxidation based on neoteric solvents and copper complexes of ditopic ligands. Dalton Trans. 2012, 41, 8285–8289. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Hayashi, M. Novel N,N-bidentate ligands for enantioselective copper(I)-catalyzed allylic oxidation of cyclic olefins. Adv. Synth. Catal. 2008, 350, 2639–2644. [Google Scholar] [CrossRef]
- Alvarez, L.X.; Christ, M.L.; Sorokin, A.B. Selective oxidation of alkenes and alkynes catalyzed by copper complexes. Appl. Catal. A Gen. 2007, 325, 303–308. [Google Scholar] [CrossRef]
- Walling, C.; Zavitsas, A.A. The Copper-Catalyzed Reaction of Peresters with Hydrocarbons. J. Am. Chem. Soc. 1963, 85, 2084–2090. [Google Scholar] [CrossRef]
- Kochi, J.K.; Mains, H.E. Studies on the Mechanism of the Reaction of Peroxides and Alkenes with Copper Salts. J. Org. Chem. 1965, 30, 1862–1872. [Google Scholar] [CrossRef]
- Beckwith, A.L.J.; Zavitsas, A.A. Allylic Oxidations by Peroxy Esters Catalyzed by Copper Salts. The Potential for Stereoselective Syntheses. J. Am. Chem. Soc. 1986, 108, 8230–8234. [Google Scholar] [CrossRef]
- Andrus, M.B.; Argade, A.B.; Chen, X.; Pamment, M.G. The asymmetric kharasch reaction. Catalytic enantioselective allylic acyloxylation of olefins with chiral copper(I) complexes and tert-butyl perbenzoate. Tetrahedron Lett. 1995, 36, 2945–2948. [Google Scholar] [CrossRef]
- Smith, K.; Hupp, C.D.; Allen, K.L.; Slough, G.A. Catalytic allylic amination versus allylic oxidation: A mechanistic dichotomy. Organometallics 2005, 24, 1747–1755. [Google Scholar] [CrossRef]
- Mayoral, J.A.; Rodríguez-Rodríguez, S.; Salvatella, L. Theoretical insights into enantioselective catalysis: The mechanism of the Kharasch-Sosnovsky reaction. Chem. Eur. J. 2008, 14, 9274–9285. [Google Scholar] [CrossRef]
- Beck, A.; Weibert, B.; Burzlaff, N. Monoanionic N,N,O-scorpionate ligands and their iron(II) and zinc(II) complexes: Models for mononuclear active sites of non-heme iron oxidases and zinc enzymes. Eur. J. Inorg. Chem. 2001, 521–527. [Google Scholar] [CrossRef]
- Otero, A.; Fernandez-Baeza, J.; Tejeda, J.; Antinolo, A.; Carrillo-Hermosilla, F.; Diez-Barra, E.; Lara-Sanchez, A.; Fernandez-Lopez, M.; Lanfranchi, M.; Pellinghelli, M.A. Syntheses and crystal structures of lithium and niobium complexes containing a new type of monoanionic “scorpionate” ligand. J. Chem. Soc. Dalton Trans. 1999, 3537–3539. [Google Scholar] [CrossRef]
- Burzlaff, N. Tripodal N,N,O-ligands for metalloenzyme models and organometallics. In Advances in Inorganic Chemistry; Academic Press: Cambridge, MA, USA, 2008; Volume 60, pp. 101–165. [Google Scholar]
- Honrado, M.; Sobrino, S.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Garcés, A.; Lara-Sánchez, A.; Rodríguez, A.M. Synthesis of an enantiopure scorpionate ligand by a nucleophilic addition to a ketenimine and a zinc initiator for the isoselective ROP of rac-lactide. Chem. Commun. 2019, 55, 8947–8950. [Google Scholar] [CrossRef] [PubMed]
- Burzlaff, N.; Hegelmann, I.; Weibert, B. Bis(pyrazol-1-yl)acetates as tripodal “scorpionate” ligands in transition metal carbonyl chemistry: Syntheses, structures and reactivity of manganese and rhenium carbonyl complexes of the type [LM(CO)3] (L = bpza, bdmpza). J. Organomet. Chem. 2001, 626, 16–23. [Google Scholar] [CrossRef]
- Paul, T.; Rodehutskors, P.M.; Schmidt, J.; Burzlaff, N. Oxygen Atom Transfer Catalysis with Homogenous and Polymer-Supported N,N- and N,N,O-Heteroscorpionate Dioxidomolybdenum(VI) Complexes. Eur. J. Inorg. Chem. 2016, 2595–2602. [Google Scholar] [CrossRef]
- Fischer, N.V.; Türkoglu, G.; Burzlaff, N. Scorpionate complexes suitable for enzyme inhibitor studies. Curr. Bioact. Compd. 2009, 5, 277–295. [Google Scholar] [CrossRef]
- Costas, M.; Mehn, M.P.; Jensen, M.P.; Que, L., Jr. Dioxygen Activation at Mononuclear Nonheme Iron Active Sites: Enzymes, Models, and Intermediates. Chem. Rev. 2004, 104, 939–986. [Google Scholar] [CrossRef] [PubMed]
- Parkin, G. Synthetic Analogues Relevant to the Structure and Function of Zinc Enzymes. Chem. Rev. 2004, 104, 699–767. [Google Scholar] [CrossRef] [PubMed]
- Pellei, M.; Bagnarelli, L.; Luciani, L.; Del Bello, F.; Giorgioni, G.; Piergentili, A.; Quaglia, W.; De Franco, M.; Gandin, V.; Marzano, C.; et al. Synthesis and Cytotoxic Activity Evaluation of New Cu(I) Complexes of Bis(pyrazol-1-yl) Acetate Ligands Functionalized with an NMDA Receptor Antagonist. Int. J. Mol. Sci. 2020, 21, 2616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giorgetti, M.; Tonelli, S.; Zanelli, A.; Aquilanti, G.; Pellei, M.; Santini, C. Synchrotron radiation X-ray absorption spectroscopic studies in solution and electrochemistry of a nitroimidazole conjugated heteroscorpionate copper(II) complex. Polyhedron 2012, 48, 174–180. [Google Scholar] [CrossRef]
- Pellei, M.; Papini, G.; Trasatti, A.; Giorgetti, M.; Tonelli, D.; Minicucci, M.; Marzano, C.; Gandin, V.; Aquilanti, G.; Dolmella, A.; et al. Nitroimidazole and glucosamine conjugated heteroscorpionate ligands and related copper(II) complexes. Syntheses, biological activity and XAS studies. Dalton Trans. 2011, 40, 9877–9888. [Google Scholar] [CrossRef]
- Morelli, M.B.; Amantini, C.; Santoni, G.; Pellei, M.; Santini, C.; Cimarelli, C.; Marcantoni, E.; Petrini, M.; Del Bello, F.; Giorgioni, G.; et al. Novel antitumor copper(ii) complexes designed to act through synergistic mechanisms of action, due to the presence of an NMDA receptor ligand and copper in the same chemical entity. New J. Chem. 2018, 42, 11878–11887. [Google Scholar] [CrossRef]
- Pellei, M.; Gandin, V.; Cimarelli, C.; Quaglia, W.; Mosca, N.; Bagnarelli, L.; Marzano, C.; Santini, C. Syntheses and biological studies of nitroimidazole conjugated heteroscorpionate ligands and related Cu(I) and Cu(II) complexes. J. Inorg. Biochem. 2018, 187, 33–40. [Google Scholar] [CrossRef]
- Reiss, A.; Cioateră, N.; Dobrițescu, A.; Rotaru, M.; Carabet, A.C.; Parisi, F.; Gănescu, A.; Dăbuleanu, I.; Spînu, C.I.; Rotaru, P. Bioactive Co(II), Ni(II), and Cu(II) Complexes Containing a Tridentate Sulfathiazole-Based (ONN) Schiff Base. Molecules 2021, 26, 3062. [Google Scholar] [CrossRef]
- Otero, A.; Fernández-Baeza, J.; Lara-Sánchez, A.; Sánchez-Barba, L.F. Metal complexes with heteroscorpionate ligands based on the bis(pyrazol-1-yl)methane moiety: Catalytic chemistry. Coord. Chem. Rev. 2013, 257, 1806–1868. [Google Scholar] [CrossRef]
- Godau, T.; Bleifuß, S.M.; Müller, A.L.; Roth, T.; Hoffmann, S.; Heinemann, F.W.; Burzlaff, N. Cu(i) catalysed cyclopropanation with enantiopure scorpionate type ligands derived from (+)-camphor or (−)-menthone. Dalton Trans. 2011, 40, 6547–6554. [Google Scholar] [CrossRef]
- Jun, Z.; Braunstein, P.; Hor, T.S.A. Highly selective chromium(III) ethylene trimerization catalysts with [NON] and [NSN] heteroscorpionate ligands. Organometallics 2008, 27, 4277–4279. [Google Scholar]
- Zhang, J.; Li, A.; Hor, T.S.A. Crystallographic revelation of the role of AIMe3 (in MAO) in Cr [NNN] pyrazolyl catalyzed ethylene trimerization. Organometallics 2009, 28, 2935–2937. [Google Scholar] [CrossRef]
- Otero, A.; Fernandez, B.; Antinolo, A.; Carrillo, H.; Tejeda, J.; Diez, B.; Lara, S.; Sanchez, B.; Lopez, S.; Ribeiro, M.R.; et al. Polymerization of ethylene by the electrophilic heteroscorpionate-containing complexes [TiCl3(bdmpza)] and [TiCl2(bdmpza) {O(CH2)4Cl}] (bdmpza = bis(3,5-dimethylpyrazol-1-yl)acetate). Organometallics 2001, 20, 2428–2430. [Google Scholar] [CrossRef]
- Türkoglu, G.; Tampier, S.; Strinitz, F.; Heinemann, F.W.; Hübner, E.; Burzlaff, N. Ruthenium Carbonyl Complexes Bearing Bis(pyrazol-1-yl)carboxylato Ligands. Organometallics 2012, 31, 2166–2174. [Google Scholar] [CrossRef]
- Rhinehart, J.L.; Manbeck, K.A.; Buzak, S.K.; Lippa, G.M.; Brennessel, W.W.; Goldberg, K.I.; Jones, W.D. Catalytic Arene H/D Exchange with Novel Rhodium and Iridium Complexes. Organometallics 2012, 31, 1943–1952. [Google Scholar] [CrossRef]
- Kopf, H.; Holzberger, B.; Pietraszuk, C.; Huebner, E.; Burzlaff, N. Neutral Ruthenium Carbene Complexes bearing N,N,O Heteroscorpionate Ligands: Syntheses and Activity in Metathesis Reactions. Organometallics 2008, 27, 5894–5905. [Google Scholar] [CrossRef]
- Datta, A.; Das, K.; Beyene, B.B.; Garribba, E.; Gajewska, M.J.; Hung, C.-H. EPR interpretation and electrocatalytic H2 evolution study of bis(3,5-di-methylpyrazol-1-yl)acetate anchored Cu(II) and Mn(II) complexes. Mol. Catal. 2017, 439, 81–90. [Google Scholar] [CrossRef]
- Tada, M.; Iwasawa, Y. Advanced chemical design with supported metal complexes for selective catalysis. Chem. Commun. 2006, 2833–2844. [Google Scholar] [CrossRef]
- Dioos, B.M.L.; Vankelecom, I.F.J.; Jacobs, P.A. Aspects of immobilisation of catalysts on polymeric supports. Adv. Synth. Catal. 2006, 348, 1413–1446. [Google Scholar] [CrossRef]
- Burguete, M.I.; Fraile, J.M.; García, J.I.; García-Verdugo, E.; Herrerías, C.I.; Luis, S.V.; Mayoral, J.A. Bis(oxazoline)copper complexes covalently bonded to insoluble support as catalysts in cyclopropanation reactions. J. Org. Chem. 2001, 66, 8893–8901. [Google Scholar] [CrossRef] [Green Version]
- Padnya, P.; Shibaeva, K.; Arsenyev, M.; Baryshnikova, S.; Terenteva, O.; Shiabiev, I.; Khannanov, A.; Boldyrev, A.; Gerasimov, A.; Grishaev, D.; et al. Catechol-Containing Schiff Bases on Thiacalixarene: Synthesis, Copper (II) Recognition, and Formation of Organic-Inorganic Copper-Based Materials. Molecules 2021, 26, 2334. [Google Scholar] [CrossRef]
- Hübner, E.; Haas, T.; Burzlaff, N. Synthesis and transition metal complexes of novel N,N,O scorpionate ligands suitable for solid phase immobilisation. Eur. J. Inorg. Chem. 2006, 4989–4997. [Google Scholar] [CrossRef]
- Hübner, E.; Türkoglu, G.; Wolf, M.; Zenneck, U.; Burzlaff, N. Novel N,N,O scorpionate ligands and transition metal complexes thereof suitable for polymerisation. Eur. J. Inorg. Chem. 2008, 1226–1235. [Google Scholar] [CrossRef]
- Türkoglu, G.; Pubill Ulldemolins, C.; Müller, R.; Hübner, E.; Heinemann, F.W.; Wolf, M.; Burzlaff, N. Bis(3,5-dimethyl-4-vinylpyrazol-1-yl)acetic Acid: A New Heteroscorpionate Building Block for Copolymers that Mimic the 2-His-1-carboxylate Facial Triad. Eur. J. Inorg. Chem. 2010, 2962–2974. [Google Scholar] [CrossRef]
- Mal, D.D.; Kundu, J.; Pradhan, D. CuO{001} as the Most Active Exposed Facet for Allylic Oxidation of Cyclohexene via a Greener Route. ChemCatChem 2021, 13, 362–372. [Google Scholar] [CrossRef]
- Tetour, D.; Novotná, M.; Hodačová, J. Enantioselective Henry Reaction Catalyzed by Copper(II) Complex of Bis(trans-cyclohexane-1,2-diamine)-Based Ligand. Catalysts 2021, 11, 41. [Google Scholar] [CrossRef]
- Sekar, G.; Dattagupta, A.; Singh, V.K. Asymmetric Kharasch Reaction: Catalytic Enantioselective Allylic Oxidation of Olefins Using Chiral Pyridine Bis(diphenyloxazoline)—Copper Complexes and tert-Butyl Perbenzoate. J. Org. Chem. 1998, 63, 2961–2967. [Google Scholar] [CrossRef]
- Gabrielli, S.; Pellei, M.; Venditti, I.; Fratoddi, I.; Battocchio, C.; Iucci, G.; Schiesaro, I.; Meneghini, C.; Palmieri, A.; Marcantoni, E.; et al. Development of new and efficient copper(II) complexes of hexyl bis(pyrazolyl)acetate ligands as catalysts for allylic oxidation. Dalton Trans. 2020, 49, 15622–15632. [Google Scholar] [CrossRef]
- ECHA European Chemicals Agency. Phenylhydrazine. Available online: https://echa.europa.eu/it/registration-dossier/-/registered-dossier/14149/7/3/1 (accessed on 12 October 2021).
- Samadi, S.; Ashouri, A.; Majidian, S.; Rashid, H. Synthesis of new alkenyl iodobenzoate derivatives via Kharasch-Sosnovsky reaction using tert-butyl iodo benzoperoxoate and copper (I) iodide. J. Chem. Sci. 2020, 132, 1–9. [Google Scholar] [CrossRef]
- Sadjadi, S.; Samadi, S.; Samadi, M. Cu(CH3CN)4PF6 immobilized on halloysite as efficient heterogeneous catalyst for oxidation of allylic C–H bonds in olefins under mild reaction condition. Res. Chem. Intermed. 2019, 45, 2441–2455. [Google Scholar] [CrossRef]
- Samadi, S.; Jadidi, K.; Samadi, M.; Ashouri, A.; Notash, B. Designing chiral amido-oxazolines as new chelating ligands devoted to direct Cu-catalyzed oxidation of allylic C H bonds in cyclic olefins. Tetrahedron 2019, 75, 862–867. [Google Scholar] [CrossRef]
- Chelucci, G.; Loriga, G.; Murineddu, G.; Pinna, G.A. Synthesis and application in asymmetric copper(I)-catalyzed allylic oxidation of a new chiral 1,10-phenanthroline derived from pinene. Tetrahedron Lett. 2002, 43, 3601–3604. [Google Scholar] [CrossRef]
- Johnson, C.K. ORTEP, Report ORNL–5138; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1976. [Google Scholar]
- Kozlevčar, B.; Pregelj, T.; Pevec, A.; Kitanovski, N.; Costa, J.S.; Van Albada, G.; Gamez, P.; Reedijk, J. Copper complexes with the ligand methyl bis(3,5-dimethylpyrazol-1-yl)- acetate (Mebdmpza), generated by in situ methanolic esterification of bis(3,5-dimethylpyrazol-1-yl)acetic acid. Eur. J. Inorg. Chem. 2008, 4977–4982. [Google Scholar] [CrossRef]
- Quillian, B.; Lynch, W.E.; Padgett, C.W.; Lorbecki, A.; Petrillo, A.; Tran, M. Syntheses and Crystal Structures of Copper(II) Bis(pyrazolyl)acetic Acid Complexes. J. Chem. Crystallogr. 2019, 49, 1–7. [Google Scholar] [CrossRef]
- Romero, M.A.; Salas, J.M.; Quiros, M.; Sanchez, M.P.; Romero, J.; Martin, D. Structural and magnetic studies on a bromine-bridged copper(II) dimer with 5,7-dimethyl[1,2,4]triazolo[1,5-a]pyrimidine. Inorg. Chem. 1994, 33, 5477–5481. [Google Scholar] [CrossRef]
- Lavrenova, L.G.; Ikorskij, V.N.; Sheludyakova, L.A.; Naumov, D.Y.; Boguslavskij, E.G. Co(II), Ni(II) and Cu(II) complexes with 1-(4-hydroxyphenyl)-1H-1,2,4-triazole. Russ. J. Coord. Chem. 2004, 30, 442–448. [Google Scholar] [CrossRef]
- Dobrzanska, L.; Kleinhans, D.J.; Barbour, L.J. Influence of the metal-to-ligand ratio on the formation of metal organic complexes. New J. Chem. 2008, 32, 813–819. [Google Scholar] [CrossRef]
- Hoffmann, A.; Herres-Pawlis, S. Dissection of Different Donor Abilities Within Bis(pyrazolyl)pyridinylmethane Transition Metal Complexes. Z. Anorg. Allg. Chem. 2013, 639, 1426–1432. [Google Scholar] [CrossRef]
- Andreeva, T.N.; Lyakhov, A.S.; Ivashkevich, L.S.; Voitekhovich, S.V.; Grigoriev, Y.V.; Ivashkevich, O.A. 1-(Furan-2-ylmethyl)-1H-tetrazole and its Copper(II) Complexes. Z. Anorg. Allg. Chem. 2015, 641, 2312–2320. [Google Scholar] [CrossRef]
- Dyukova, I.I.; Kuz’menko, T.A.; Komarov, V.Y.; Sukhikh, T.S.; Vorontsova, E.V.; Lavrenova, L.G. Coordination Compounds of Cobalt(II), Nickel(II), and Copper(II) Halides with 2-Methyl-1,2,4-Triazolo[1,5-a]benzimidazole. Russ. J. Coord. Chem. 2018, 44, 755–764. [Google Scholar] [CrossRef]
- Allen, F.H. The Cambridge Structural Database: A quarter of a million crystal structures and rising. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Konno, T.; Tokuda, K.; Sakurai, J.; Okamoto, K.-I. Five-Coordinate Geometry of Cadmium(II) with Octahedral Bidentate-S,S Complex-Ligand cis(S)-[Co(aet)2(en)]+ (aet = 2-aminoethanethiolate): Synthesis, Crystal Structures and Interconversion of S-Bridged CoIIICdII Polynuclear Complexes. Bull. Chem. Soc. Jpn. 2000, 73, 2767–2773. [Google Scholar] [CrossRef]
- CrysAlisPro 1.171.41.103a Rigaku Oxford Diffraction. 2021. Available online: https://www.rigaku.com/products/crystallography/crysalis (accessed on 17 September 2021).
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]






| Entry | 1 mol% | 2:3 Ratio | Reaction Time (h) | Yield (%) a 4 |
|---|---|---|---|---|
| 1a | 5 | 5:1 | 6 | 73 |
| 1b | 5 | 5:1 | 24 | 95 |
| 1c | 5 | 3:1 | 24 | 79 |
| 1d | 1 | 5:1 | 24 | 77 |
| 1e | 1 | 3:1 | 24 | 74 |
| 1f | 0.5 | 10:1 | 24 | 95 |
| 1g | 0.5 | 5:1 | 24 | 90 |
| 1h | 0.5 | 3:1 | 24 | 90 |
| 1i | 0.5 | 1:1 | 48 | 33 |
| Catalyst Type | Reaction Conditions | Yield (%) | Ref. | |
|---|---|---|---|---|
 | Cu(I) and Cu(II) salts | 5 mol% cat., 32–48 h, PhNHNH2 (6 μL), CH3CN (3 mL), reflux | 85 (n = 1) 92 (n = 2) 76 (n = 4) | [76] |
| Cu(CH3CN)4PF6 immobilized on halloysite | 20 mg cat., 7–45 h, CH3CN (3 mL), r.t. | 67 (n = 1) 90 (n = 2) 80 (n = 4) | [77] | |
| Cu(I) and Cu(II) complexes | 2.5 mol% Cu salt, 5 mol% chiral ligand, 50–81 h, PhNHNH2 (2 μL), HZSM-5 (5 mg), acetone, 0 °C | 75 (n = 1) 85 (n = 3) 75 (n = 4) | [78] | |
| Cu(II) complexes | 1 mol% Cu(OTf)2, 1.1 mol% chiral ligand, 0.5–168 h, 1.1 mol% PhNHNH2, acetone, r.t. | 78 (n = 1) 82 (n = 2) 81 (n = 3) 0 (n = 4) | [79] | |
| CuBr | 10 mol% CuBr, 18 h, dichlorobenzene, 70 °C, inert atm. | 70 (n = 2) | [9] |
| Bond | Bond Length (Å) | Bond | Bond Length (Å) |
| Br(1)−Cu(1) | 2.4302 (4) | Br(2)−Cu(1) | 2.3687 (6) |
| Br(1)−Cu(1) I | 2.7600 (5) | Cu(1)−N(1) | 2.017 (2) |
| Cu(1)−N(3) | 2.042 (3) | N(1)−N(2) | 1.357 (3) |
| N(3)−N(4) | 1.369 (3) | N(1)−C(3) | 1.330 (4) |
| N(2)−C(1) | 1.342 (4) | N(3)−C(6) | 1.305 (4) |
| N(4)−C(4) | 1.338 (4) | N(2)−C(7) | 1.445 (3) |
| N(4)−C(7) | 1.434 (4) | C(1)−C(2) | 1.344 (5) |
| C(2)−C(3) | 1.384 (5) | C(4)−C(5) | 1.355 (5) |
| C(5)−C(6) | 1.399 (5) | C(7)−C(8) | 1.533 (4) |
| O(1)−C(8) | 1.198 (3) | O(2)−C(8) | 1.310 (4) |
| Bond | Bond Angle (°) | Bond | Bond Angle (°) |
| Cu(1)−Br(1)−Cu(1) I | 93.544 (13) | Br(1)−Cu(1)−Br(1) I | 86.456 (13) |
| N(1)−Cu(1)−Br(1) I | 93.87 (7) | N(3)−Cu(1)−Br(1) I | 97.29 (7) |
| Br(2)−Cu(1)−Br(1) | 93.260 (18) | N(1)−Cu(1)−Br(1) | 176.35 (7) |
| N(3)−Cu(1)−Br(2) | 157.34 (7) | N(1)−Cu(1)−N(3) | 85.69 (10) |
| N(1)−Cu(1)−Br(2) | 90.16 (8) | N(3)−Cu(1)−Br(1) | 90.67 (7) |
| N(2)−N(1)−Cu(1) | 122.68 (17) | N(4)−N(3)−Cu(1) | 121.3 (2) |
| N(1)−N(2)−C(7) | 118.8 (2) | N(3)−N(4)−C(7) | 119.4 (2) |
| N(1)−C(3)−C(2) | 110.6 (3) | N(3)−C(6)−C(5) | 111.5 (3) |
| C(1)−N(2)−N(1) | 111.2 (2) | C(4)−N(4)−N(3) | 110.6 (3) |
| N(2)−C(1)−C(2) | 107.3 (3) | N(4)−C(4)−C(5) | 107.9 (3) |
| N(4)−C(7)−N(2) | 110.1 (2) | C(1)−C(2)−C(3) | 106.2 (3) |
| C(4)−C(5)−C(6) | 104.8 (4) | O(2)−C(8)−C(7) | 108.8 (2) |
| O(1)−C(8)−C(7) | 124.6 (3) | O(1)−C(8)−O(2) | 126.6 (3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagnarelli, L.; Dolmella, A.; Santini, C.; Vallesi, R.; Giacomantonio, R.; Gabrielli, S.; Pellei, M. A New Dimeric Copper(II) Complex of Hexyl Bis(pyrazolyl)acetate Ligand as an Efficient Catalyst for Allylic Oxidations. Molecules 2021, 26, 6271. https://doi.org/10.3390/molecules26206271
Bagnarelli L, Dolmella A, Santini C, Vallesi R, Giacomantonio R, Gabrielli S, Pellei M. A New Dimeric Copper(II) Complex of Hexyl Bis(pyrazolyl)acetate Ligand as an Efficient Catalyst for Allylic Oxidations. Molecules. 2021; 26(20):6271. https://doi.org/10.3390/molecules26206271
Chicago/Turabian StyleBagnarelli, Luca, Alessandro Dolmella, Carlo Santini, Riccardo Vallesi, Roberto Giacomantonio, Serena Gabrielli, and Maura Pellei. 2021. "A New Dimeric Copper(II) Complex of Hexyl Bis(pyrazolyl)acetate Ligand as an Efficient Catalyst for Allylic Oxidations" Molecules 26, no. 20: 6271. https://doi.org/10.3390/molecules26206271
APA StyleBagnarelli, L., Dolmella, A., Santini, C., Vallesi, R., Giacomantonio, R., Gabrielli, S., & Pellei, M. (2021). A New Dimeric Copper(II) Complex of Hexyl Bis(pyrazolyl)acetate Ligand as an Efficient Catalyst for Allylic Oxidations. Molecules, 26(20), 6271. https://doi.org/10.3390/molecules26206271