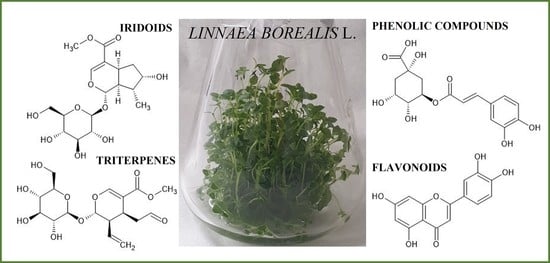
Linnaea borealis L. var. borealis—In Vitro Cultures and Phytochemical Screening as a Dual Strategy for Its Ex Situ Conservation and a Source of Bioactive Compounds of the Rare Species
Abstract
:1. Introduction
2. Results
2.1. Shoot Multiplication
2.2. Shoot Rooting
2.3. Genome Size Estimation
2.4. Callus Induction and Maintenance
2.5. UPLC-HESI-HRMS Phytochemical Screening
3. Discussion
4. Materials and Methods
4.1. Plant Material, In Vitro Cultures Initiation, and Growth Chamber Parameters
4.2. Shoot Multiplication on Solid Media
4.3. Shoot Multiplication in Liquid Media (Agitated Cultures)
4.4. Shoot Rooting
4.5. Callus Induction and Maintenance
4.6. Flow Cytometry
4.7. UPLC-HESI-HRMS Phytochemical Screening
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Backlund, A.; Pyck, N. Diervillaceae and Linnaeaceae, two new families of caprifolioids. Taxon 1998, 47, 657–661. [Google Scholar] [CrossRef]
- Alm, T. Ethnobotany of Linnaea borealis (Linnaeaceae) in Norway. Bot. J. Linn. Soc. 2006, 151, 437–452. [Google Scholar] [CrossRef] [Green Version]
- Landrein, S.; Farjon, A. A monograph of Caprifoliaceae: Linnaeeae. Kew Bull. 2019, 74, 70. [Google Scholar] [CrossRef]
- Hulten, E. Flora of Alaska and Neighboring Territories: A Manual of the Vascular Plants; Stanford University Press: Stanford, CA, USA, 1986. [Google Scholar]
- Niva, M. Life History Strategies in Linnaea borealis. Ph.D. Thesis, Uppsala University, Uppsala, Sweden, 2003; Volume 893; pp. 1–21.
- Wiberg, R.A.W.; Scobie, A.R.; A’Hara, S.W.; Ennos, R.A.; Cottrell, J.E. The genetic consequences of long term habitat fragmentation on a self-incompatible clonal plant, Linnaea borealis L. Biol. Conserv. 2016, 201, 405–413. [Google Scholar] [CrossRef] [Green Version]
- Foster, J.; Burnett, S.; Stack, L. Effects of light, soil moisture, and nutrition on greenhouse propagation of Twinflower. Hort. Technol. 2017, 27, 782–788. [Google Scholar] [CrossRef] [Green Version]
- Mirek, Z.; Piękoś-Mirkowa, H.; Zając, A.; Zając, M. Flowering Plants and Pteridophytes of Poland. A Check List; Krytyczna Lista Roślin Naczyniowych Polski; Instytut Botaniki PAN im. Władysława Szafera w Krakowie: Kraków, Poland, 2020; ISBN 83-85444-83-1. [Google Scholar]
- Zawadzka, D.; Zawadzki, G.; Bednarek, J.; Bednarek, J.B.; Piechowska, D.; Mikitiuk, A. The twinflower in the Augustow Forest: Occurrence, condition and threats. For. Res. Pap. 2017, 78, 77–78. [Google Scholar] [CrossRef] [Green Version]
- Thiem, B.; Buk-Berge, E. Twinflower (Linnaea borealis L.)—Plant species of potential medicinal properties. Herba Pol. 2017, 63, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Glennie, C.W. Comparative Phytochemical Study of Caprifoliaceae. Ph.D. Thesis, The University of British Columbia, Vancouver, BC, Canada, 1969; pp. 3–96. [Google Scholar]
- Bergström, L.G.W.; Bergquist, S.; Stenhagen, G.; Gahmberg, C.G.; Maia, A.C.D.; Nordenstam, B. Floral scent chemistry within the genus Linnaea (Caprifoliaceae). Nord. J. Bot. 2018, e01732. [Google Scholar] [CrossRef]
- NAED. Native American Ethnobotany Database: BRIT. A Database of Foods, Drugs, Dyes and Fibres of Native American Peoples Derived from Plants. 2021. Available online: http://naeb.brit.org (accessed on 2 August 2021).
- Brondegaard, V.J. Linnaea i folkemedicine. Sven. Linnésällskapets Årsskrift 1959, 42, 89–98. (In Norwegian) [Google Scholar]
- Smetanska, I. Production of secondary metabolites using plant cell cultures. Adv. Biochem. Eng. Biotechnol. 2008, 111, 187–228. [Google Scholar] [CrossRef] [PubMed]
- Chandana, B.C.; Kumari Nagaveni, H.C.; Heena, M.S.; Kolakar, S.S.; Lakshmana, D. Role of plant tissue in micropropagation, secondary metabolites production and conservation of some endangered medicinal crops. J. Pharmacogn. Phytochem. 2018, SP3, 246–251. [Google Scholar]
- Kikowska, M.; Thiem, B.; Szopa, A.; Klimek-Szczykutowicz, M.; Rewers, M.; Sliwinska, E.; Ekiert, H. Comparative analysis of phenolic acids and flavonoids in shoot cultures of Eryngium alpinum L.: An endangered and protected species with medicinal value. Plant Cell Tiss. Organ Cult. 2019, 139, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Biswas, T.; Dwivedi, U.N. Plant triterpenoid saponins: Biosynthesis, in vitro production, and pharmacological relevance. Protoplasma 2019, 256, 1463–1486. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Wang, C.; Gong, X.; Bo, A.; Zhang, L.; Zhang, M.; Zhang, E.; Li, M. Iridoids: Research Advances in their Phytochemistry, Biological Activities, and Pharmacokinetics. Molecules 2020, 25, 287. [Google Scholar] [CrossRef] [Green Version]
- Ramabulana, A.T.; Steenkamp, P.; Madala, N.; Dubery, I.A. Profiling of chlorogenic acids from Bidenspilosa and differentiation of closely related positional isomers with the aid of UHPLC-QTOF-MS/MS-based in-source collision-induced dissociation. Metabolites 2020, 10, 178. [Google Scholar] [CrossRef]
- Kucharska, A.Z.; Fecka, I. Identification of Iridoids in Edible Honeysuckle Berries (Lonicera caerulea L. var. kamtschatica Sevast.) by UPLC-ESI-qTOF-MS/MS. Molecules 2016, 21, 1157. [Google Scholar] [CrossRef] [Green Version]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef]
- Qi, Q.; Guo, C.; Zhang, Q. Multiple shoots induction and rapid propagation of Lonicera edulis. In Proceedings of the AASRI International Conference on Industrial Electronics and Applications (IEA, 2015), London, UK, 27–28 June 2015; 2015; pp. 695–699. [Google Scholar] [CrossRef] [Green Version]
- Dziedzic, E. Propagation of Blue Honeysuckle (Lonicera caerulea var. kamtschatica Pojark.) in in vitro culture. J. Fruit Ornam. Plant Res. 2008, 16, 93–100. [Google Scholar]
- Boonnour, K.; Wainwright, H.; Hicks, R.G.T. The micropropagation of Lonicera periclymenum L. (Honysucle). Acta Hortic. 1988, 226, 183–190. [Google Scholar] [CrossRef]
- Thiem, B.; Kikowska, M.; Krawczyk, A.; Więckowska, B.; Sliwinska, E. Phenolic acid and DNA contents of micropropagated Eryngium planum L. Plant Cell Tiss. Organ Cult. 2013, 114, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Kikowska, M.; Thiem, B.; Sliwinska, E.; Rewers, M.; Kowalczyk, M.; Stochmal, A.; Oleszek, W. The effect of nutritional factors and plant growth regulators on micropropagation and production of phenolic acids and saponins from plantlets and adventitious root cultures of Eryngium maritimum L. J. Plant Growth Regul. 2014, 33, 809–819. [Google Scholar] [CrossRef] [Green Version]
- Kikowska, M.; Thiem, B.; Sliwinska, E.; Rewers, M.; Kowalczyk, M.; Stochmal, A.; Długaszewska, J. Micropropagation of Eryngium campestre L. via shoot culture provides valuable uniform plant material with enhanced content of phenolic acids and antimicrobial activity. Acta Biol. Cracov. Ser. Bot. 2016, 58, 43–56. [Google Scholar] [CrossRef]
- LiYing, D. Study on rapid propagation of dormant bud of Kolkwitzia amabilis. J. Henan Agric. Sci. 2014, 43, 99–102. [Google Scholar]
- Wang, X.; Li, Y.; Zeng, H.; Cai, N.; Qiao, Z.; Wang, X. Micropropagation of Weigela florida ‘Tango’ through in vitro shoot culture. HortScience 2017, 52, 274–277. [Google Scholar] [CrossRef] [Green Version]
- Maliński, M.P.; Kikowska, M.; Kruszka, D.; Napierała, M.; Florek, E.; Sliwinska, E.; Thiem, B. Various in vitro systems of Ragged Robin (Lychnis flos-cuculi L.): A new potential source of phytoecdysteroids? Plant Cell Tiss. Organ Cult. 2019, 139, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Grzegorczyk-Karolak, I.; Rytczak, P.; Bielecki, S.; Wysokińska, H. The influence of liquid systems for shoot multiplication, secondary metabolite production and plant regeneration of Scutellaria alpina. Plant Cell Tiss. Organ Cult. 2017, 128, 479–486. [Google Scholar] [CrossRef] [Green Version]
- Szopa, A.; Kokotkiewicz, A.; Marzec-Wróblewska, U.; Buciński, A.; Luczkiewicz, M.; Ekiert, H. Accumulation of dibenzocyclooctadiene ligans in agar cultures and in stationary and agitated liquid cultures of Schisandra chinensis (Turcz.) Baill. Appl. Microbiol. Biotechnol. 2016, 100, 3965–3977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karhu, S.T. Rooting of blue honeysuckle microshoots. Plant Cell Tiss. Organ Cult. 1997, 48, 153–159. [Google Scholar] [CrossRef]
- Sedlak, J.; Paprstein, F. In vitro propagation of blue honeysuckle. Hortic. Sci. 2007, 34, 129–131. [Google Scholar] [CrossRef] [Green Version]
- Mujib, A.; Pipal, T.; Ali, M.; Tonk, D.; Zafar, N.; Gulzar, B. In vitro propagation of Althaea officinalis: The role of plant growth regulators in morphogenesis. Biotechnologia 2017, 98, 167–173. [Google Scholar] [CrossRef]
- Kikowska, M.; Budzianowski, J.; Krawczyk, A.; Thiem, B. Accumulation of rosmarinic, chlorogenic and caffeic acids in in vitro cultures of Eryngium planum L. Acta Physiol. Plant. 2012, 34, 2425–2433. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, S.R.; Bowers, M.D. Iridoid and secoiridoid glycosides in a hybrid complex of bush honeysuckles (Lonicera spp., Caprifolicaceae): Implications for evolutionary ecology and invasion biology. Phytochemistry 2013, 86, 57–63. [Google Scholar] [CrossRef]
- Jensen, S.R.; Nielsen, B.J.; Dahlgren, R. Iridoid compounds, their occurrence and systematic importance in the angiosperms. Bot. Notiser 1975, 128, 148–180. [Google Scholar]
- Mao, Q.; Cao, D.; Jia, X.S. Studies on the chemical constituents of Lonicera macranthoides Hand.-Mazz. Acta Pharm. Sin. 1993, 28, 273–281. [Google Scholar]
- Chen, Y.; Feng, X.; Jia, X.D.; Wang, M.; Liang, J.Y.; Dong, Y.F. Triterpene glycosides from Lonicera. Isolation and structural determination of seven glycosides from flower buds of Lonicera macranthoides. Chem. Nat. Compd. 2008, 44, 9–43. [Google Scholar] [CrossRef]
- Kwak, W.J.; Han, C.K.; Chang, H.W.; Kim, H.P.; Kang, S.S.; Son, K.H. Loniceroside C, an anti-inflammatory saponin from Lonicera japonica. Chem. Pharm. Bull. 2003, 51, 333–335. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.W.; Jung, H.A.; Kang, S.S.; Choi, J.S. Antioxidant constituents and a new triterpenoid glycoside from floslonicerae. Arch. Pharm. Res. 2007, 30, 1–7. [Google Scholar] [CrossRef]
- Xiang, T.; Wu, L.J.; Zheng, L.; Wu, B.; Men, T.Z.L.; He, M. Three new saponins from Lonicera bournei Hemsl. J. Shenyang Pharm. Univ. 2000, 17, 215. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Marie, D.; Brown, S.C. A cytometric exercise in plant histograms, with 2C values for 70 species. Biol. Cell 1993, 78, 41–51. [Google Scholar] [CrossRef]
- Galbraith, D.W.; Harkins, K.R.; Maddox, J.M.; Ayres, N.M.; Sharma, D.P.; Firoozabady, E. Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 1983, 220, 1049–1051. [Google Scholar] [CrossRef] [PubMed]




| MS Medium Supplementation | Explants (Mean No. of New Shoots ± SE) | ||||
|---|---|---|---|---|---|
| Cytokinin (mg/L) | Auxin (mg/L) | Gibberellin (mg/L) | Double Shoots | Single Shoot with Apical Meristem | Shoot Segments with One Node |
| - | - | - | 2.46 ± 0.22 cd | 2.00 ± 0.17 c | 5.46 ± 0.31 ab |
| BAP 1.0 | IAA 0.1 | - | 4.15 ± 0.28 bc | 3.00 ± 0.19 b | 5.67 ± 0.36 ab |
| BAP 0.5 | IAA 0.1 | GA3 1.0 | 3.19 ± 0.50 cd | 2.75 ± 0.25 b | 5.25 ± 0.33 abc |
| BAP 1.0 | IAA 0.1 | GA3 1.0 | 4.95 ± 1.49 b | 2.58 ± 0.15 bc | 3.92 ± 0.36 d |
| BAP 1.5 | IAA 0.1 | GA3 1.0 | 5.12 ± 0.71 b | 3.25 ± 0.25 b | 6.13 ± 0.64 a |
| BAP 2.0 | IAA 0.1 | GA3 1.0 | 7.63 ± 0.73 a | 4.10 ± 0.32 a | 4.50 ± 0.61 bcd |
| Kin 1.0 | IAA 0.1 | GA3 1.0 | 2.16 ± 0.22 c | 2.58 ± 0.19 bc | 4.07 ± 0.40 cd |
| Kin 2.0 | IAA 0.1 | GA3 1.0 | 2.39 ± 0.20 c | 2.67 ± 0.19 bc | 6.33 ± 0.43 a |
| Explants | Number of New Shoots per Explant ± SE | Number of New Shoots per One Shoot ± SE |
|---|---|---|
| 1/single shoot | 5.63 ± 0.63 b | 5.63 ± 0.63 a |
| 2/double shoot | 10.89 ± 0.55 a | 5.37 ± 0.26 a |
| 3/triple shoot | 10.79 ± 0.53 a | 3.51 ± 0.20 b |
| Explants | Number of New Shoots per Explant ± SE | Number of New Shoots per One Shoot ± SE | Shoots Length Increase (%) LI ± SE |
|---|---|---|---|
| 1/single shoot | 17.46 ± 0.91 b | 17.46 ± 0.91 b | 154.77 ± 15.16 a |
| 2/double shoot | 48.80 ± 3.67 a | 24.11 ± 1.77 a | 150.36 ± 19.33 a |
| 3/triple shoot | 50.30 ± 5.25 a | 15.72 ± 1.75 b | 138.14 ± 8.25 b |
| In Vitro System | Explants | Number of New Shoots per Explant ± SE | Number of New Shoots per One Shoot ± SE |
|---|---|---|---|
| Solid medium | 1/single shoot | 5.63 ± 0.63 d | 5.63 ± 0.63 c |
| Liquid medium | 1/single shoot | 17.46 ± 0.91 b | 17.46 ± 0.91 b |
| Solid medium | 2/double shoot | 10.89 ± 0.55 c | 5.37 ± 0.26 c |
| Liquid medium | 2/double shoot | 48.80 ± 3.67 a | 24.11 ± 1.77 a |
| Solid medium | 3/triple shoot | 10.79 ± 0.53 c | 3.51 ± 0.20 d |
| Liquid medium | 3/triple shoot | 50.30 ± 5.25 a | 15.72 ± 1.75 b |
| Medium | IAA (mg/L) | IBA (mg/L) | Induction (%) | Root Number ± SE | Root Length (cm) ± SE |
|---|---|---|---|---|---|
| ¼ MS | - | - | 50 | 4.57 ± 0.91 de | 2.01 ± 0.11 b |
| ½ MS | - | - | 50 | 5.14 ± 1.03 de | 1.56 ± 0.08 cde |
| MS | - | - | 30.77 | 1.50 ± 0.50 e | 1.32 ± 0.26 efg |
| MS | 1.0 | - | 38.46 | 1.60 ± 0.40 e | 0.95 ± 0.19 gh |
| MS | 2.0 | - | 76.92 | 2.50 ± 0.50 e | 1.96 ± 0.13 bc |
| MS | 3.0 | - | 76.92 | 4.44 ± 0.85 de | 1.94 ± 0.14 bc |
| MS | 4.0 | - | 33.33 | 3.25 ± 0.63 e | 1.44 ± 0.21 def |
| MS | 2.0 | 5.0 | 94.74 | 17.22 ± 1.91 ab | 0.99 ± 0.04 gh |
| MS | 2.0 | 0.5 | 76.92 | 4.60 ± 0.91 de | 2.45 ± 0.13 a |
| MS | 1.0 | 2.0 | 100 | 19.78 ± 2.62 a | 0.67 ± 0.03 h |
| MS | 1.0 | 1.0 | 50 | 4.13 ± 0.83 de | 1.76 ± 0.10 bcd |
| MS | 1.0 | 0.5 | 50 | 2.00 ± 0.82 e | 1.68 ± 0.25 bcde |
| MS | 0.5 | 2.0 | 100 | 16.00 ± 2.97 ab | 0.91 ± 0.06 gh |
| MS | - | 1.0 | 100 | 10.00 ± 1.15 cd | 0.97 ± 0.05 fg |
| MS | - | 2.0 | 100 | 16.08 ± 2.25 ab | 1.17 ± 0.05 fg |
| MS | - | 3.0 | 100 | 13.36 ± 1.36 bc | 1.91 ± 0.07 bc |
| MS | - | 4.0 | 100 | 19.30 ± 1.86 a | 1.00 ± 0.04 gh |
| Plant Material | No. of Samples | DNA Content (pg/2C ± SE) |
|---|---|---|
| Seedling (Control) | 3 | 1.778 ± 0.011 ns |
| Plantlets (S1–S2) | 12 | 1.783 ± 0.021 |
| MS Medium Supplementation | Callus Growth Index (%) ± SE | ||
|---|---|---|---|
| Passage VII | Passage VIII | Passage IX | |
| Pic 1.0 mg/L | 379.67 ± 45.51 ab | 485.78 ± 21.77 a | 345.61 ± 21.61 b |
| Pic 2.0 mg/L | 408.99 ± 31.24 a | 468.66 ± 10.64 a | 525.80 ± 18.46 a |
| 2,4-D 2.0 mg/L + NAA 0.5 mg/L | 281.40 ± 35.18 b | 311.56 ± 23.67 b | 380.83 ± 26.74 b |
| No | rt (min) | [M − H]− | [M + H]+ | Name | Plant Material | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UPLC-MS | UPLC-PDA | m/z | d (ppm) | Formula | Fragmentation | m/z | d (ppm) | Formula | Fragmentation | ||||||
| C | R | SC | NC | ||||||||||||
| 1 | 2.23 | 1.13 | 191.0550 | −2.74 | C7H11O6− | nd | nd | nd | Quinic acid (A) | + | + | + | + | ||
| 2 | 4.53 | 3.43 | 218.1028 | 0.49 | C9H17NO5− | 146.0816, 88.0390, 71.0121 | nd | nd | Pantothenic acid (A) | + | + | + | + | ||
| 3 | 3.32 | 2.22 | 577.1349 | 0.50 | C30H25O12− | 407.0768, 289.0717, 245.0813, 125.0229 | 579.1490 | 2.18 | C30H27O12 | 409.0899, 287.0527, 127.0391 | Procyanidin (F) | nd | + | + | + |
| 4 | 3.49 | 2.39 | 577.1348 | 1.50 | C30H25O12− | 407.0768, 289.0717, 245.0813, 125.0229 | 579.1493 | 1.56 | C30H27O12 | 409.0899, 287.0527, 127.0391 | Procyanidin (F) | nd | nd | + | + |
| 5 | 4.23 | 3.13 | 289.0718 | 2.17 | C15H13O6− | 245.0816, 179.0340, 151.0388, 125.0228, 109.0279 | 291.0853 | 5.38 | C15H15O6 | 207.0646, 139.0392, 123.0430 | Catechin (F) | nd | nd | + | + |
| 6 | 4.58 | 3.48 | 609.1462 | 0.50 | C27H29O16− | 447.0927, 285.0402 | 611.1589 | 3.84 | C27H31O16 | 287.0554 | Luteolin-3′,7-di-O-glucoside (F) | + | + | + | + |
| 7 | 4.71 | 3.61 | 577.1344 | 0.60 | C30H25O12− | 407.0768, 289.0717, 245.0813, 125.0229 | 579.1494 | 1.56 | C30H27O12 | 409.0899, 287.0527, 127.0391 | Procyanidin (F) | nd | nd | + | + |
| 8 | 5.76 | 4.66 | 609.1459 | 0.63 | C27H29O16− | 301.0353, 300.0275 | 611.1594 | 2.94 | C27H31O16 | 303.0471 | Quercetin-3-O-rutinoside (F) | nd | + | + | + |
| 9 | 5.94 | 4.84 | 593.1516 | 1.53 | C27H29O15− | 285.0403, 269.0450 | 595.1666 | −0.55 | C27H31O15 | nd | Luteolin-7-O-rhamnoglucoside I (F) | nd | + | + | + |
| 10 | 6.02 | 4.92 | 463.0887 | 2.29 | C21H19O12− | 301.03519, 300.02737 | 465.1028 | 1.06 | C21H21O12 | 303.0471 | Quercetin-3-O-glucoside (F) | nd | + | + | + |
| 11 | 6.23 | 5.13 | 593.1516 | 1.80 | C27H29O15− | 285.0403, 284.0323 | 595.1669 | −1.06 | C27H31O15 | 287.0554 | Kaempferol-3-O-rutinoside (F) | nd | + | + | + |
| 12 | 6.25 | 5.15 | 463.0887 | 2.29 | C21H19O12− | 301.03519, 300.02737 | 465.1028 | 1.06 | C21H21O12 | 303.0471 | Quercetin-3-O-galactoside (F) | nd | + | + | + |
| 13 | 6.11 | 5.01 | 447.0928 | 1.30 | C21H19O11− | 285.0404, 284.0328 | 449.1079 | 1.20 | C21H21O11 | 287.0554 | Kaempferol-3-O-glucoside (F) | nd | nd | + | + |
| 14 | 6.34 | 5.24 | 563.1409 | 1.51 | C26H27O14− | 269.0455, 133.0274 | 565.1566 | −1.57 | C26H29O14 | 433.1159, 271.0610 | Apigenin-7-O- apioglucoside | + | nd | + | + |
| 15 | 6.72 | 5.62 | 447.0928 | 1.30 | C21H19O11− | 285.0413, 133.0381 | 449.1079 | 1.20 | C21H21O11 | 287.0554 | Luteoloin-7-O-glucoside (F) | nd | + | + | + |
| 16 | 6.71 | 5.61 | 593.1515 | 1.90 | C27H29O15− | 285.0403, 269.04623, 257.04605, 151.00226 | 595.1669 | −1.01 | C27H31O15 | 287.0554 | Luteoloin-O- rhamnoglucoside II (F) | nd | + | + | + |
| 17 | 6.50 | 5.40 | 577.1574 | 0.70 | C27H29O14− | 269.0454 | 579.1696 | 3.15 | C27H31O14 | 271.0609 | Apigenin-7-O- rhamnoglucoside (F) | nd | + | + | + |
| 18 | 6.80 | 5.70 | 431.0981 | 1.74 | C21H19O10− | 269.0455 | 433.1129 | 1.26 | C21H21O10 | 271.061 | Apigenin-7-O-glucoside (F) | nd | + | + | + |
| 19 | 7.98 | 6.88 | 447.0928 | 1.30 | C21H19O11− | 285.0403 | 449.1078 | 1.20 | C21H21O11 | 287.0554 | Luteolin-7-O-glucoside (F) | nd | nd | + | + |
| 20 | 8.13 | 7.03 | 285.0404 | 3.77 | C15H9O6− | 241.0508, 217.0504, 175.0383, 151.0023, 133.0281 | 287.0542 | 4.65 | C15H11O6 | 153.0199, 137.0958, 135.0938 | Luteolin (F) | nd | + | + | + |
| 21 | 9.10 | 8.00 | 269.0457 | 4.57 | C15H9O5− | 151.002 | 271.0592 | 5.35 | C15H11O5 | 153.0175, 119.0488 | Apigenin (F) | nd | + | + | + |
| 22 | 9.26 | 8.16 | 285.0405 | 3.98 | C15H9O6− | 270.2250, 257.0460, 151.0026 | 287.0544 | 4.02 | C15H11O6 | nd | Kaempferol (F) | nd | nd | + | + |
| 23 | 9.42 | 8.32 | 299.0558 | 0.86 | C16H11O6− | 284.0325 | 301.0699 | 4.37 | C16H13O6 | nd | Kaempferide (F) | nd | + | + | + |
| 24 | 4.09 | 2.99 | 373.1138 | 2.27 | C16H21O10− | 211.0604, 193.0493, 167.0701, 149.0594, 123.0436 | 375.1276 | 4.07 | C16H23O10 | 213.0742, 195.0644, 177.0546, 167.0704 | Swertiamarin (I) | nd | nd | + | + |
| 25 | 4.11 | 3.01 | 375.1294 | 0.72 | C16H23O10− | 213.0761, 169.0858, 151.0752, 125.0752, 119.0335, 113.0228 | 377.1439 | 2.21 | C16H25O10 | 215.0914, 197.0428, 179.0694, 151.0756, 109.0650 | Loganic acid (I) | nd | + | + | + |
| 26 | 4.27 | 3.17 | 375.1294 | 0.72 | C16H23O10− | 213.0764, 195.0652, 151.0751 | 377.1433 | 3.83 | C16H25O10 | 215.0914, 197.0428, 153.0537, 127.0391, 111.0806 | Epi-loganic acid (I) | nd | + | + | + |
| 27 | 4.21 | 3.11 | 435.1508 | 1.13 | C18H27O12− [M + FA − H]− | 227.0918, 191.0550, 127.0385, 101.0227 | 391.1591 | 3.39 | C17H27O10 | 229.1074, 211.0972, 179.0709, 151.0395, 109.0650 | Loganin (I) | nd | + | + | + |
| 28 | 4.62 | 3.52 | 373.1138 | 2.51 | C16H21O10− | 267.0658, 239.0716, 211.0753, 193.0495, 149.0594 | 375.1279 | 3.35 | C16H23O10 | 213.0743, 195.0642, 177.0546, 167.0712, 151.0388, 149.0600, 133.0288, 125.0233, 107.0493 | Geniposidic acid (I) | nd | + | + | + |
| 29 | 5.14 | 4.04 | 403.1246 | 1.47 | C17H23O11− | 223.0595, 165.0545, 121.0279 | 427.1203 | 3.19 | C17H24O11Na | 265.0668, 255.0840, 233.0420, 195.0270 | Secoxyloganin (I) | + | + | + | + |
| 30 | 5.16 | 4.06 | 357.1192 | 1.80 | C16H21O9− | 195.06508, 125.02290 | 381.1151 | 2.77 | C16H22O9Na | 255.0829, 219.0623, 185.0404, 149.0203 | Sweroside (I) | nd | + | + | + |
| 31 | 5.47 | 4.37 | 403.1247 | 1.72 | C17H23O11− | nd | 405.1379 | 4.42 | C17H25O11 | 211.0967, 193.0843, 177.0546, 161.0598, 151.0389 | Gardenoside (I) | nd | + | + | + |
| 32 | 5.81 | 4.71 | 387.1298 | 1.80 | C17H23O10− | 225.0759, 193.0496, 181.0494, 155.0336, 123.0435, 113.0228, 101.0228 | 389.1437 | 2.76 | C17H25O10 | 209.0801, 177.0543, 165.0547, 151.0386, 107.0493 | Secologanin (I) | nd | + | + | + |
| 33 | 5.97 | 4.87 | 359.1349 | 1.98 | C16H23O9− | nd | 361.1484 | 4.15 | C16H25O9 | nd | Deoxyloganic acid (I) | nd | + | + | + |
| 34 | 7.15 | 6.05 | 537.1614 | 1.16 | C25H29O13− | 375.1288, 179.0338, 161.0231, 135.0436 | nd | nd | nd | Grandifloroside (I) | + | + | + | + | |
| 35 | 7.54 | 6.44 | 585.2195 | 1.96 | C27H37O14− | 373.1134, 211.09676, 193.0497, 149.05943 | nd | nd | nd | Unknown iridoid I (I) | nd | nd | + | + | |
| 36 | 7.98 | 6.88 | 583.2036 | 1.59 | C27H35O14− | 373.1143, 209.0815, 193.0497, 149.0595 | nd | nd | nd | Unknown iridoid II (I) | nd | + | + | + | |
| 37 | 3.43 | 2.33 | 299.0771 | 1.30 | C13H15O8− | 137.0229, 93.0329 | 301.0920 | 1.20 | C13H17O8 | nd | Hydroxybenzoic acid hexoside I (P) | + | + | + | + |
| 38 | 3.58 | 2.48 | 359.0981 | 3.44 | C15H19O10− | 197.0446, 182.0211, 153.0544, 138.0308 | nd | nd | Dimethoxy-hydroxybenzoic acid hexoside (P) | nd | + | + | + | ||
| 39 | 3.67 | 2.57 | 315.0723 | 2.20 | C13H15O9− | 153.0544, 123.0436, 109.0279 | nd | nd | Dihydroxybenzoic acid hexoside (P) | + | + | + | + | ||
| 40 | 3.95 | 2.85 | 341.0875 | 2.80 | C15H17O9− | 179.0339, 135.0437 | nd | nd | Caffeic acid hexoside (P) | nd | + | + | + | ||
| 41 | 3.81 | 2.71 | 299.0771 | 1.30 | C13H15O8− | 137.0229 | nd | nd | Hydroxybenzoic acid hexoside II (P) | + | + | + | + | ||
| 42 | 3.92 | 2.82 | 353.0879 | 3.25 | C16H17O9− | 191.0551, 179.0339, 161.0233, 135.0437 | 355.1021 | 2.28 | C16H19O9 | 163.0389, 145.0283, 135.0439, 117.0342 | 3-Caffeoylquinic acid (P) | + | + | + | + |
| 43 | 3.34 | 2.24 | 339.0718 | 2.31 | C15H15O9− | 177.0182 | 341.0879 | −1.91 | C15H17O9 | 179.0331, 151.0758 | Esculin (P) | nd | nd | + | + |
| 44 | 4.36 | 3.26 | 353.0875 | 3.25 | C16H17O9− | 191.0551 | 355.1022 | 2.08 | C16H19O9 | 163.0389, 145.0283, 135.0439 | 5-Caffeoylquinic acid (P) | + | + | + | + |
| 45 | 4.88 | 3.78 | 353.0875 | 3.25 | C16H17O9− | 191.0551, 179.0339, 173.04428, 161.0233, 135.0437 | 355.1021 | 2.42 | C16H19O9 | 163.0389, 145.0283, 135.0439 | 4-Caffeoylquinic acid (P) | + | + | + | + |
| 46 | 4.93 | 3.83 | 353.0875 | 3.25 | C16H17O9− | 191.0551 | 355.1022 | 2.06 | C16H19O9 | 163.0389, 145.0283, 135.0439 | Cis-5-Caffeoylquinic acid (P) | nd | + | + | + |
| 47 | 4.99 | 3.89 | 337.0929 | 3.26 | C16H17O8− | 191.0551 | 339.1069 | 3.23 | C16H19O8 | 195.0641, 177.0547, 165.0539, 147.0437, 119.0491 | 3-Coumaroyl quinic acid (P) | nd | + | + | + |
| 48 | 5.36 | 4.26 | 367.1038 | 2.32 | C17H19O9− | 193.0497, 191.0551, 173.0444 | 369.1175 | 2.90 | C17H21O9 | 177.0546, 145.0283, 117.033 | Feruloylquinic acid (P) | nd | + | + | + |
| 49 | 5.49 | 4.39 | 337.0930 | 3.26 | C16H17O8− | 191.0551 | 339.1071 | 2.58 | C16H19O8 | 147.0437, 119.0491, 91.0542 | 5-Coumaroyl quinic acid (P) | nd | + | + | + |
| 50 | 5.77 | 4.67 | 367.1038 | 2.32 | C17H19O9− | 191.0551 | 369.1173 | 3.47 | C17H21O9 | 177.0546, 135.0439 | Feruloylquinic acid (P) | nd | + | + | + |
| 51 | 6.33 | 5.23 | 515.1201 | 2.10 | C25H23O12− | 353.0883, 335.0776, 179.0338, 173.0446, 161.0229, 135.0436 | 517.1340 | 2.76 | C25H25O12 | 163.0389, 145.0283, 135.0439 | 3,4-Caffeoylquinic acid (P) | nd | + | + | + |
| 52 | 6.46 | 5.36 | 515.1191 | 1.55 | C25H23O12− | 353.0876, 353.0876, 191.0551, 179.0339, 173.0444 | 517.1335 | 2.18 | C25H25O12 | 163.0389, 145.0283, 135.0439 | 3,4-Caffeoylquinic acid (P) | nd | + | + | + |
| 53 | 6.58 | 5.48 | 515.1191 | 0.36 | C25H23O12− | 353.0877, 191.0551, 179.0339, 135.0437 | 517.1334 | 2.41 | C25H25O12 | 163.0389, 145.0283, 135.0439 | 3,5-Caffeoylquinic acid (P) | nd | + | + | + |
| 54 | 6.56 | 5.46 | 579.2079 | 1.89 | C28H35O13− | 417.1554, 402.1318, 387.1084, 181.0495, 166.0259 | nd | nd | S(8-8)S hexoside (P) | nd | + | + | + | ||
| 55 | 6.84 | 5.74 | 515.1191 | 0.37 | C25H23O12− | 353.0877, 191.0551, 179.0339, 135.0437 | 517.1335 | 2.18 | C25H25O12 | 163.0389, 145.0283, 135.0439 | 3,5-Caffeoylquinic acid (P) | nd | + | + | + |
| 56 | 7.57 | 6.47 | 515.1193 | 0.70 | C25H23O12− | 353.0879, 191.0551, 179.0339, 173.0444, 135.0437 | 517.1334 | 2.29 | C25H25O12 | 163.0389, 145.0283, 135.0439 | 4,5-Caffeoylquinic acid (P) | nd | + | + | + |
| 57 | 7.13 | 6.03 | 499.1249 | 1.81 | C25H23O11− | 337.0934, 173.0445, 163.0389 | nd | nd | 3-Caffeoyl-5-coumaroylquinic acid (P) | nd | + | + | + | ||
| 58 | 7.31 | 6.21 | 499.1249 | 1.81 | C25H23O11− | 353.0879, 337.0933, 191.0551, 179.0339, 163.0388, 135.0437 | nd | nd | 3-Caffeoyl-4-coumaroylquinic acid (P) | nd | + | + | + | ||
| 59 | 7.48 | 6.38 | 499.1249 | 1.81 | C25H23O11− | 353.0879, 337.0933, 191.0551, 179.0339, 173.0444, 163.0388 | nd | nd | 5-Caffeoyl-4-coumaroylquinic acid (P) | nd | + | + | + | ||
| 60 | 7.42 | 6.32 | 529.1361 | 1.81 | C26H25O12− | 367.1029, 353.0869, 193.0498, 191.0551, 179.0339, 173.0446, 135.0438 | nd | nd | 3-Caffeoyl-5-feruloylquinic acid (P) | + | + | + | + | ||
| 61 | 7.72 | 6.62 | 529.1361 | 1.81 | C26H25O12− | 367.1029, 353.0869, 193.0498, 191.0551, 179.0339, 173.0446, 135.0438 | nd | nd | 4-Caffeoyl-5-feruloylquinic acid (P) | + | + | + | + | ||
| 62 | 8.18 | 7.08 | 499.1249 | 1.81 | C25H23O11− | 353.0874, 337.0928, 191.0552, 179.0339, 173.0444 | nd | nd | 4-Caffeoyl-5-coumaroylquinic acid (P) | nd | + | + | + | ||
| 63 | 8.42 | 7.32 | 179.0338 | −3.49 | C9H7O4− | 135.0437 | 181.0494 | 3.78 | C9H9O4 | 163.0389, 145.0282, 138.0437 | Caffeic acid (P) | nd | nd | + | + |
| 64 | 9.05 | 7.95 | 515.1199 | 2.04 | C25H23O12− | 353.0879, 191.0551, 179.0339, 173.0444, 135.0437 | 517.13342 | 2.29 | C25H25O12 | nd | 4,5-Caffeoylquinic acid (P) | nd | + | + | + |
| 65 | 8.71 | 7.61 | 1235.6069 | 0.69 | C59H95O27_ | nd | nd | nd | Macranthoidin A (T) | nd | + | + | + | ||
| 66 | 9.10 | 8.00 | 927.4966 | 1.40 | C47H75O18− | 603.3903, 453.3357 | nd | nd | Akebiasaponin D (T) | nd | + | + | + | ||
| 973.5060 | 1.54 | C48H77O20− [M + FA]− | 603.3888 | nd | + | + | + | ||||||||
| 67 | 10.21 | 9.11 | 1073.5542 | 0.89 | C53H85O22− | nd | nd | nd | Loniceroside C (T) | nd | + | + | + | ||
| 68 | 11.32 | 10.22 | 957.5079 | 2.12 | C48H77O19− | 749.4479, 587.3954, 455.3544 | nd | nd | Bourneioside B (T) | nd | + | + | + | ||
| 69 | 11.83 | 10.73 | 1057.523 | 1.87 | C52H81O22− | 687.4111, 567.3679 | nd | nd | Unknown Saponin I (T) | nd | + | + | + | ||
| 70 | 12.54 | 11.44 | 911.4990 | 1.34 | C47H75O17− | 749.4475, 893.4863, 849.4981, 749.4475, 705.4574, 687.4468, 603.3901, 541.3889, 471.3448 | nd | nd | Unknown Saponin II (T) | nd | + | + | + | ||
| 71 | 12.60 | 11.50 | 765.4407 | 0.15 | C41H65O13− | 603.3917, 471.3464 | nd | nd | Cauloside C (T) | + | + | + | + | ||
| 72 | 12.60 | 11.50 | 811.4491 | 1.43 | C42H67O15− | nd | nd | nd | Unknown Saponin III (T) | nd | + | + | + | ||
| 73 | 13.21 | 12.11 | 749.4494 | 0.93 | C41H65O12− | 587.3946, 569.3834, 455.3531 | nd | nd | Alpha-Hederin (T) | nd | + | + | + | ||
| 74 | 13.85 | 12.75 | 603.3904 | 1.14 | C35H55O8− | 557.3862, 453.3366 | nd | nd | Cauloside A (T) | nd | + | + | + | ||
| 75 | 14.83 | 13.73 | 795.4532 | 1.43 | C42H67O14− | nd | nd | nd | Unknown Saponin IV (T) | nd | + | + | + | ||
| 76 | 15.14 | 14.04 | 471.3478 | 0.86 | C30H48O4− | 453.3356 | nd | nd | Hederagenin (T) | + | + | + | + | ||
| 77 | 15.66 | 14.56 | 469.3325 | 1.63 | C30H46O4− | nd | nd | nd | Gypsogenin (T) | + | + | + | + | ||
| 78 | 17.32 | 16.22 | 455.3531 | 1.25 | C30H47O3− | nd | nd | nd | Oleanonic acid (T) | + | + | + | + | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiem, B.; Kruszka, D.; Turowska, N.; Sliwinska, E.; Berge, V.; Kikowska, M. Linnaea borealis L. var. borealis—In Vitro Cultures and Phytochemical Screening as a Dual Strategy for Its Ex Situ Conservation and a Source of Bioactive Compounds of the Rare Species. Molecules 2021, 26, 6823. https://doi.org/10.3390/molecules26226823
Thiem B, Kruszka D, Turowska N, Sliwinska E, Berge V, Kikowska M. Linnaea borealis L. var. borealis—In Vitro Cultures and Phytochemical Screening as a Dual Strategy for Its Ex Situ Conservation and a Source of Bioactive Compounds of the Rare Species. Molecules. 2021; 26(22):6823. https://doi.org/10.3390/molecules26226823
Chicago/Turabian StyleThiem, Barbara, Dariusz Kruszka, Natalia Turowska, Elwira Sliwinska, Viktor Berge, and Małgorzata Kikowska. 2021. "Linnaea borealis L. var. borealis—In Vitro Cultures and Phytochemical Screening as a Dual Strategy for Its Ex Situ Conservation and a Source of Bioactive Compounds of the Rare Species" Molecules 26, no. 22: 6823. https://doi.org/10.3390/molecules26226823
APA StyleThiem, B., Kruszka, D., Turowska, N., Sliwinska, E., Berge, V., & Kikowska, M. (2021). Linnaea borealis L. var. borealis—In Vitro Cultures and Phytochemical Screening as a Dual Strategy for Its Ex Situ Conservation and a Source of Bioactive Compounds of the Rare Species. Molecules, 26(22), 6823. https://doi.org/10.3390/molecules26226823