Graphene-Based Biosensors with High Sensitivity for Detection of Ovarian Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth and Transfer of Graphene Film
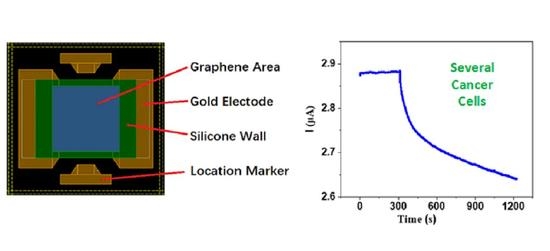
2.2. Fabrication of Graphene-Based Biosensors
2.3. Culture of SKOV3 Ovarian Cancer Cells
2.4. Preparation of Cancer Cell Solution and Electrical Test
3. Results and Discussion
3.1. Morphology and Structure of Graphene Film and Photos of Graphene-Based Biosensor
3.2. Biosensing Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Badgwell, D.; Bast, R.C., Jr. Early Detection of Ovarian Cancer. Dis. Markers 2007, 23, 397–410. [Google Scholar] [CrossRef] [Green Version]
- Bonello, M.; Sims, A.H.; Langdon, S.P. Human epidermal growth factor receptor targeted inhibitors for the treatment of ovarian cancer. Cancer Biol. Med. 2018, 15, 375–388. [Google Scholar] [CrossRef] [Green Version]
- Dood, R.L.; Zhao, Y.; Armbruster, S.D.; Coleman, R.L.; Tworoger, S.; Sood, A.K.; Baggerly, K.A. Defining Survivorship Trajectories Across Patients With Solid Tumors: An Evidence-Based Approach. JAMA Oncol. 2018, 4, 1519–1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottoni, P.; Scatena, R. The Role of CA 125 as Tumor Marker: Biochemical and Clinical Aspects. Adv. Exp. Med. Biol. 2015, 867, 229–244. [Google Scholar] [CrossRef]
- Sharma, S.; Zhuang, R.; Long, M.; Pavlovic, M.; Kang, Y.; Ilyas, A.; Asghar, W. Circulating tumor cell isolation, culture, and downstream molecular analysis. Biotechnol. Adv. 2018, 36, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Tewes, M.; Aktas, B.; Welt, A.; Mueller, S.; Hauch, S.; Kimmig, R.; Kasimir-Bauer, S. Molecular profiling and predictive value ofcirculating tumor cells in patients with metastatic breast cancer: An option for monitoring response to breast cancer related therapies. Breast Cancer Res. Treat. 2009, 115, 581–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satelli, A.; Brownlee, Z.; Mitra, A.; Meng, Q.H.; Li, S. Circulating Tumor Cell Enumeration with a Combination of Epithelial Cell Adhesion Moleculeand Cell-Surface Vimentin-based Methods for Monitoring Breast Cancer Therapeutic Response. Clin. Chem. 2015, 61, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Budd, G.T.; Cristofanilli, M.; Ellis, M.J.; Stopeck, A.; Borden, E.; Miller, M.C.; Matera, J.; Repollet, M.; Doyle, G.V.; Terstappen, L.W.; et al. Circulating Tumor Cells versusImaging—Predicting Overall Survival in Metastatic Breast Cancer. Clin. Cancer Res. 2006, 12, 6403–6409. [Google Scholar] [CrossRef] [Green Version]
- Cheung, K.J.; Ewald, A.J. A collective route to metastasis: Seeding by tumor cell clusters. Science 2016, 352, 167–169. [Google Scholar] [CrossRef] [Green Version]
- Chaffer, C.L.; San Juan, B.P.; Lim, E.; Weinberg, R.A. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016, 35, 645–654. [Google Scholar] [CrossRef]
- Lee, M.; Kim, E.J.; Cho, Y.; Kim, S.; Chung, H.H.; Park, N.H.; Song, Y.S. Predictive value of circulating tumor cells (CTCs) captured by microfluidic device in patients with epithelial ovarian cancer. Gynecol. Oncol. 2017, 145, 361–365. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Hua, E.; Liang, M.; Ma, C.; Liu, Z.; Sheng, S.; Liu, M.; Xie, G.; Feng, W. Graphene sheets, polyaniline and aunts based DNA sensor for electrochemical determination of BCR/ABL fusion gene with functional hairpin probe. Biosens. Bioelectron. 2014, 51, 201–207. [Google Scholar] [CrossRef]
- Samanman, S.; Numnuam, A.; Limbut, W.; Kanatharana, P.; Thavarungkul, P. Highly-sensitive label-free electrochemical carcinoembryonic antigen immune-sensor based on a novel aunanoparticles-graphene-chitosan nanocomposite cryogen electrode. Anal. Chem. Acta 2015, 853, 521–532. [Google Scholar] [CrossRef]
- Satelli, A.; Mitra, A.; Cutrera, J.J.; Xia, X.; Li, S. Abstract 1469: Specific detection tool for mesenchymal and epithelial-mesenchymal transformed circulating tumor cells. Cancer Res. 2014, 73 (Suppl. S8), 1469. [Google Scholar] [CrossRef]
- Li, T.; Li, Z.; Zhou, J.; Pan, B.; Xiao, X.; Guo, Z.; Wu, L.; Chen, Y. Outlook and Challenges of Nano Devices, Sensors, and MEMS||The Application of Graphene in Biosensors; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 299–329. [Google Scholar] [CrossRef]
- Li, P.; Zhang, B.; Cui, T. Towards intrinsic graphene biosensor: A label-free, suspended singlecrystalline graphene sensor for multiplex lung cancer tumor markers detection. Biosens. Bioelectron. 2015, 72, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Gao, W.; Gupta, B.K.; Liu, Z.; Romero-Aburto, R.; Ge, L.; Song, L.; Alemany, L.B.; Zhan, X.; Gao, G.; et al. Graphene quantum dots derived from carbon fibers. Nano Lett. 2012, 12, 844–849. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Z.; Chen, Y.; Liu, J.; Zheng, B.; Zhang, W.; Li, Y. Growth and properties of large-area sulfur-doped graphene films. J. Mater. Chem. C 2017, 5, 7944–7949. [Google Scholar] [CrossRef]
- Li, X.S.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [Green Version]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Wang, Z.G.; Chen, Y.F.; Li, P.J.; Hao, X.; Liu, J.B.; Huang, R.; Li, Y.R. Flexible Graphene-Based Electroluminescent Devices. ACS Nano 2011, 5, 7149–7154. [Google Scholar] [CrossRef]
- Liu, J.; Li, P.; Chen, Y.; Wang, Z.; He, J.; Tian, H.; Qi, F.; Zheng, B.; Zhou, J.; Lin, W. Large-area synthesis of high-quality and uniform monolayer graphene without unexpected bilayer regions. J. Alloys Compd. 2014, 615, 415–418. [Google Scholar] [CrossRef]
- Oh, S.; Kim, C.K.; Kim, J. High Responsivity beta-Ga2O3 Metal-Semiconductor-Metal Solar-Blind Photodetectors with Ultraviolet Transparent Graphene Electrodes. ACS Photonics 2018, 5, 1123–1128. [Google Scholar] [CrossRef]
- Zheng, B.J.; Chen, Y.F.; Wang, Z.G.; Qi, F.; Huang, Z.S.; Hao, X.; Li, P.J.; Zhang, W.L.; Li, Y.R. Vertically oriented few-layered HfS2 nanosheets: Growth mechanism and optical properties. 2D Materials 2016, 3, 035024. [Google Scholar] [CrossRef]
- Zheng, B.J.; Wang, Z.G.; Chen, Y.F.; Zhang, W.L.; Li, X.S. Centimeter-sized 2D alpha-MoO3 single crystal: Growth, Raman anisotropy, and optoelectronic properties. 2D Materials 2018, 5, 045011. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, Q.; Han, L.; Guo, Y.; Yu, H.; Tan, L.; Zheng, A.; Chen, Y. Graphene-Based Biosensors with High Sensitivity for Detection of Ovarian Cancer Cells. Molecules 2021, 26, 7265. https://doi.org/10.3390/molecules26237265
Wan Q, Han L, Guo Y, Yu H, Tan L, Zheng A, Chen Y. Graphene-Based Biosensors with High Sensitivity for Detection of Ovarian Cancer Cells. Molecules. 2021; 26(23):7265. https://doi.org/10.3390/molecules26237265
Chicago/Turabian StyleWan, Qi, Ling Han, Yunchuan Guo, Huijun Yu, Li Tan, Ai Zheng, and Yali Chen. 2021. "Graphene-Based Biosensors with High Sensitivity for Detection of Ovarian Cancer Cells" Molecules 26, no. 23: 7265. https://doi.org/10.3390/molecules26237265
APA StyleWan, Q., Han, L., Guo, Y., Yu, H., Tan, L., Zheng, A., & Chen, Y. (2021). Graphene-Based Biosensors with High Sensitivity for Detection of Ovarian Cancer Cells. Molecules, 26(23), 7265. https://doi.org/10.3390/molecules26237265