Probing Molybdenum Active Sites during In Situ Photoreduction of the Mo6+/SiO2 Catalyst
Abstract
:1. Introduction
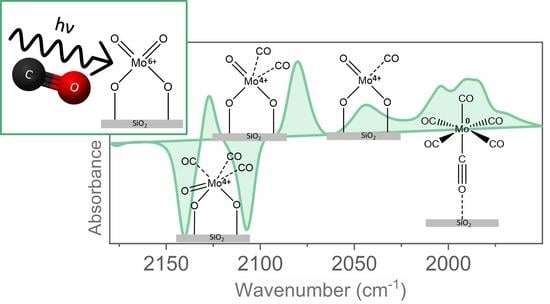
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of the Mo/SiO2 Catalyst
3.2. Pretreatment Conditions and Spectroscopic Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Haber, J. Chapter 10—Molybdenum Compounds in Heterogeneous Catalysis. In Studies in Inorganic Chemistry; Braithwaite, E.R., Haber, J., Eds.; Elsevier: Amsterdam, The Netherlands, 1994; Volume 19, pp. 477–617. [Google Scholar]
- Ohler, N.; Bell, A.T. Selective oxidation of methane over MoOx/SiO2: Isolation of the kinetics of reactions occurring in the gas phase and on the surfaces of SiO2 and MoOx. J. Catal. 2005, 231, 115–130. [Google Scholar] [CrossRef]
- Lee, E.L.; Wachs, I.E. In situ spectroscopic investigation of the molecular and electronic structures of SiO2 supported surface metal oxides. J. Phys. Chem. C 2007, 111, 14410–14425. [Google Scholar] [CrossRef]
- Banares, M.A.; Hu, H.C.; Wachs, I.E. Molybdena on silica catalysts: Role of preparation methods on the structure-selectivity properties for the oxidation of methanol. J. Catal. 1994, 150, 407–420. [Google Scholar] [CrossRef]
- Amakawa, K.; Sun, L.; Guo, C.; Havecker, M.; Kube, P.; Wachs, I.E.; Lwin, S.; Frenkel, A.I.; Patlolla, A.; Hermann, K.; et al. How strain affects the reactivity of surface metal oxide catalysts. Angew. Chem. Int. Ed Engl. 2013, 52, 13553–13557. [Google Scholar] [CrossRef] [PubMed]
- Anpo, M.; Kondo, M.; Coluccia, S.; Louis, C.; Che, M. Application of dynamic photoluminescence spectroscopy to the study of the active surface sites on supported Mo/SiO2 catalysts: Features of anchored and impregnated catalysts. J. Am. Chem. Soc. 1989, 111, 8791–8799. [Google Scholar] [CrossRef]
- Chempath, S.; Zhang, Y.; Bell, A.T. DFT Studies of the structure and vibrational spectra of isolated molybdena species supported on silica. J. Phys. Chem. C 2007, 111, 1291–1298. [Google Scholar] [CrossRef]
- Kurleto, K.; Tielens, F.; Handzlik, J. Isolated molybdenum(VI) and tungsten(VI) oxide species on partly dehydroxylated silica: A computational perspective. J. Phys. Chem. C 2020, 124, 3002–3013. [Google Scholar] [CrossRef]
- Amakawa, K.; Wang, Y.Q.; Krohnert, J.; Schlogl, R.; Trunschke, A. Acid sites on silica-supported molybdenum oxides probed by ammonia adsorbtion: Experiment and theory. Mol. Catal. 2019, 478, 9. [Google Scholar] [CrossRef]
- Mestl, G.; Srinivasan, T.K.K. Raman spectroscopy of monolayer-type catalysts: Supported molybdenum oxides. Catal. Rev. 1998, 40, 451–570. [Google Scholar] [CrossRef]
- Guesmi, H.; Grybos, R.; Handzlik, J.; Tielens, F. Characterization of molybdenum monomeric oxide species supported on hydroxylated silica: A DFT study. Phys. Chem. Chem. Phys. 2014, 16, 18253–18260. [Google Scholar] [CrossRef]
- Gholampour, N.; Yusubov, M.; Verpoort, F. Investigation of the preparation and catalytic activity of supported Mo, W, and Re oxides as heterogeneous catalysts in olefin metathesis. Catal. Rev. 2016, 58, 113–156. [Google Scholar] [CrossRef]
- Wachs, I.E.; Segawa, K. Supported metal oxide. In Characterization of Catalytic Materials, 2nd ed.; Wachs, I.E., Ed.; Momentum Press LLC: New York, NY, USA, 2010; pp. 69–88. [Google Scholar]
- Shelimov, B.N.; Pershin, A.N.; Kazansky, V.B. Selective photoreduction of molybdenum ions supported on silica. J. Catal. 1980, 64, 426–436. [Google Scholar] [CrossRef]
- Shelimov, B.N.; Elev, L.V.; Kazansky, V.B. Use of photoreduction for activation of silica-molybdena catalysts for propylene metathesis: Comparison with thermal reduction. J. Catal. 1986, 98, 70–81. [Google Scholar] [CrossRef]
- Kazansky, V.B.; Shelimov, B.N. Supported silica-molybdena catalysts for olefin metathesis activated by photoreduction. Res. Chem. Intermed. 1991, 15, 1–16. [Google Scholar] [CrossRef]
- Aigler, J.M.; Houalla, M.; Hercules, D.M. Surface structure and metathesis activity of photoreduced allyl-based Mo/SiO2 catalysts. Top. Catal. 2000, 10, 123–126. [Google Scholar] [CrossRef]
- Lwin, S.; Wachs, I.E. Olefin metathesis by supported metal oxide catalysts. ACS Catal. 2014, 4, 2505–2520. [Google Scholar] [CrossRef]
- Mino, L.; Spoto, G.; Bordiga, S.; Zecchina, A. Particles morphology and surface properties as investigated by HRTEM, FTIR, and Periodic DFT calculations: From pyrogenic TiO2 (P25) to nanoanatase. J. Phys. Chem. C 2012, 116, 17008–17018. [Google Scholar] [CrossRef]
- Zaera, F. New advances in the use of infrared absorption spectroscopy for the characterization of heterogeneous catalytic reactions. Chem. Soc. Rev. 2014, 43, 7624–7663. [Google Scholar] [CrossRef]
- Mino, L. IR spectroscopy as a tool to investigate photocatalytic reactions at oxide surfaces. Rend. Lincei. Sci. Fis. Nat. 2017, 28, 143–149. [Google Scholar] [CrossRef]
- Guglielminotti, E.; Giamello, E. Spectroscopic characterization of a molybdena silica system photoreduced in a carbon-monoxide atmosphere. J. Chem. Soc. Faraday Trans. I 1985, 81, 2307–2322. [Google Scholar] [CrossRef]
- Gerasimov, S.F. CO and NO adsorption on photoreduced Mo/SiO2 catalysts. React. Kinet. Catal. Lett. 1986, 32, 275–280. [Google Scholar] [CrossRef]
- Rodrigo, L.; Marcinkowska, K.; Roberge, P.C.; Kaliaguine, S. Photoreducibility of Mo/SiO2 catalysts with CO. J. Catal. 1987, 107, 8–22. [Google Scholar] [CrossRef]
- Kamegawa, T.; Takeuchi, R.; Matsuoka, M.; Anpo, M. Photocatalytic oxidation of CO with various oxidants by Mo oxide species highly dispersed on SiO2 at 293K. Catal. Today 2006, 111, 248–253. [Google Scholar] [CrossRef]
- Toyao, T.; Morishima, J.; Saito, M.; Horiuchi, Y.; Kamegawa, T.; Martra, G.; Coluccia, S.; Matsuoka, M.; Anpo, M. FT-IR study of the reaction mechanisms for photocatalytic reduction of NO with CO promoted by various single-site photocatalysts. J. Catal. 2013, 299, 232–239. [Google Scholar] [CrossRef]
- Williams, C.C.; Ekerdt, J.G. Infrared spectroscopic characterization of molybdenum carbonyl species formed by ultraviolet photoreduction of silica-supported Mo(VI) in carbon-monoxide. J. Phys. Chem. 1993, 97, 6843–6852. [Google Scholar] [CrossRef]
- Mino, L.; Zecchina, A.; Martra, G.; Rossi, A.M.; Spoto, G. A surface science approach to TiO2 P25 photocatalysis: An in situ FTIR study of phenol photodegradation at controlled water coverages from sub-monolayer to multilayer. Appl. Catal. B Environ. 2016, 196, 135–141. [Google Scholar] [CrossRef]
- Barzan, C.; Mino, L.; Morra, E.; Groppo, E.; Chiesa, M.; Spoto, G. Photoinduced ethylene polymerization on titania nanoparticles. ChemCatChem 2017, 9, 4324–4327. [Google Scholar] [CrossRef]
- Mino, L.; Barzan, C.; Martino, G.A.; Piovano, A.; Spoto, G.; Zecchina, A.; Groppo, E. Photoinduced ethylene polymerization on the CrVI/SiO2 Phillips catalyst. J. Phys. Chem. C 2019, 123, 8145–8152. [Google Scholar] [CrossRef]
- Aigler, J.M.; Kazansky, V.B.; Houalla, M.; Proctor, A.; Hercules, D.M. Photoreduction study of Mo allyl-based Mo/SiO2 catalysts. J. Phys. Chem. 1995, 99, 11489–11493. [Google Scholar] [CrossRef]
- Louis, C.; Che, M.; Anpo, M. Activation and properties of Mo=O bonds in Mo/SiO2 catalysts. Res. Chem. Intermed. 1991, 15, 81–98. [Google Scholar] [CrossRef]
- Ogata, A.; Kazusaka, A.; Enyo, M.; Toyoshima, I. Active-sites on the MoO3/SiO2 catalyst photoreduced with CO for CO2 reduction to CO. Chem. Phys. Lett. 1986, 127, 283–285. [Google Scholar] [CrossRef]
- Praliaud, H. Diffuse reflectance spectra of molybdenum ions supported by magnesia, γ-alumina or silica. J. Less Common Met. 1977, 54, 387–399. [Google Scholar] [CrossRef]
- Fournier, M.; Louis, C.; Che, M.; Chaquin, P.; Masure, D. Polyoxometallates as models for oxide catalysts: Part I. An UV-visible reflectance study of polyoxomolybdates: Influence of polyhedra arrangement on the electronic transitions and comparison with supported molybdenum catalysts. J. Catal. 1989, 119, 400–414. [Google Scholar] [CrossRef]
- Vikulov, K.A.; Shelimov, B.N.; Kazansky, V.B. IR and UV-Vis spectroscopic studies of the surface Mo=CH2 and Mo=CH-CH3 carbene complexes produced by methylcyclopropane chemisorption over photoreduced silica molybdena catalysts. J. Mol. Catal. 1991, 65, 393–402. [Google Scholar] [CrossRef]
- Williams, C.C.; Ekerdt, J.G.; Jehng, J.M.; Hardcastle, F.D.; Turek, A.M.; Wachs, I.E. A Raman and ultraviolet diffuse reflectance spectroscopic investigation of silica-supported molybdenum oxide. J. Phys. Chem. 1991, 95, 8781–8791. [Google Scholar] [CrossRef]
- Mino, L.; Mandrile, L.; Iannarelli, L.; Portesi, C.; Martra, G.; Rossi, A.M. Vibrational spectroscopy. In Characterization of Nanoparticles: Measurement Processes for Nanoparticles; Hodoroaba, V., Unger, W., Shard, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 457–480. [Google Scholar] [CrossRef]
- Mino, L.; Negri, C.; Zecchina, A.; Spoto, G. Photodegradation of organic pollutants on TiO2 P25 surfaces investigated by transmission FTIR spectroscopy under in situ UV-Vis irradiation. Z. Phys. Chem. Int. J. Res. Phys. Chem. Chem. Phys. 2016, 230, 1441–1451. [Google Scholar] [CrossRef]
- Matsuoka, M.; Kamegawa, T.; Takeuchi, R.; Anpo, M. In situ characterization of the highly dispersed Mo6+-oxide species supported onto various oxides and their photocatalytic reactivities. Catal. Today 2007, 122, 39–45. [Google Scholar] [CrossRef]
- Yoshida, Y.; Izumi, Y. Recent advances in the preferential thermal-/photo-oxidation of carbon monoxide: Noble versus inexpensive metals and their reaction mechanisms. Catal. Surv. Asia 2016, 20, 141–166. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santalucia, R.; Spoto, G.; Mino, L. Probing Molybdenum Active Sites during In Situ Photoreduction of the Mo6+/SiO2 Catalyst. Molecules 2021, 26, 1700. https://doi.org/10.3390/molecules26061700
Santalucia R, Spoto G, Mino L. Probing Molybdenum Active Sites during In Situ Photoreduction of the Mo6+/SiO2 Catalyst. Molecules. 2021; 26(6):1700. https://doi.org/10.3390/molecules26061700
Chicago/Turabian StyleSantalucia, Rosangela, Giuseppe Spoto, and Lorenzo Mino. 2021. "Probing Molybdenum Active Sites during In Situ Photoreduction of the Mo6+/SiO2 Catalyst" Molecules 26, no. 6: 1700. https://doi.org/10.3390/molecules26061700
APA StyleSantalucia, R., Spoto, G., & Mino, L. (2021). Probing Molybdenum Active Sites during In Situ Photoreduction of the Mo6+/SiO2 Catalyst. Molecules, 26(6), 1700. https://doi.org/10.3390/molecules26061700