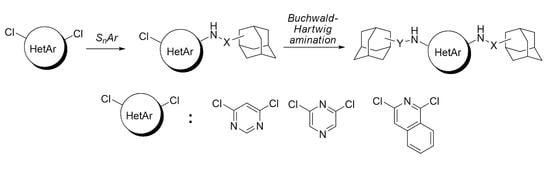
Mono- and Diamination of 4,6-Dichloropyrimidine, 2,6-Dichloropyrazine and 1,3-Dichloroisoquinoline with Adamantane-Containing Amines †
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. N-(Heteroaryl)-Substituted Adamantane-Containing Amines 5–7 (General Procedure)
3.2. Palladium-Catalyzed Amination of Chloroheterocycles—General Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Reddick, J.J.; Saha, S.; Lee, J.-M.; Melnick, J.S.; Perkins, J.; Begley, T.P. The mechanism of action of bacimethrin, a naturally occurring thiamin antimetabolite. Bioorg. Med. Chem. Lett. 2001, 11, 2245–2248. [Google Scholar] [CrossRef]
- Liang, X.; Wang, B.; Chen, C.; Wang, A.; Hu, C.; Zou, F.; Yu, K.; Liu, Q.; Li, F.; Hu, Z.; et al. Discovery of N-(4-(6-Acetamidopyrimidin-4-yloxy)phenyl)-2-(2-(trifluoromethyl)phenyl)acetamide (CHMFL-FLT3–335) as a Potent FMS-like Tyrosine Kinase 3 Internal Tandem Duplication (FLT3-ITD) Mutant Selective Inhibitor for Acute Myeloid Leukemia. J. Med. Chem. 2019, 62, 875–892. [Google Scholar] [CrossRef]
- Manz, T.D.; Sivakumaren, S.C.; Yasgar, A.; Hall, M.D.; Davis, M.I.; Seo, H.-S.; Card, J.D.; Ficarro, S.B.; Shim, H.; Marto, J.A.; et al. Structure–Activity Relationship Study of Covalent Pan-phosphatidylinositol 5-Phosphate 4-Kinase Inhibitors. ACS Med. Chem. Lett. 2020, 11, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Hong, F.; Kohm, C.; Jenkins, S.; Tulinsky, J.; Bhatt, R.; de Vries, P.; Singer, J.W.; Klein, P. Synthesis, SAR, and antitumor properties of diamino-C,N-diarylpyrimidine positional isomers: Inhibitors of lysophosphatidic acid acyltransferase-β. Bioorg. Med. Chem. Lett. 2004, 14, 2303–2308. [Google Scholar] [CrossRef] [PubMed]
- Reich, S.H.; Sprengeler, P.A.; Chiang, G.G.; Appleman, J.R.; Chen, J.; Clarine, J.; Eam, B.; Ernst, J.T.; Han, Q.; Goel, V.K.; et al. Structure-based Design of Pyridone–Aminal eFT508 Targeting Dysregulated Translation by Selective Mitogen-activated Protein Kinase Interacting Kinases 1 and 2 (MNK1/2) Inhibition. J. Med. Chem. 2018, 61, 3516–3540. [Google Scholar] [CrossRef] [PubMed]
- Mologni, L.; Dalla Via, M.; Chilin, A.; Palumbo, M.; Marzaro, G. Discovery of wtRET and V804MRET Inhibitors: From Hit to Lead. ChemMedChem 2017, 12, 1390–1398. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Lv, H.; Luo, L.; Xu, Y.; Pan, Y.; Wang, Y.; Lin, H.; Xiong, J.; Guo, P.; Zhang, J.; et al. Design, synthesis and pharmacological evaluation of N4,N6-disubstituted pyrimidine-4,6-diamine derivatives as potent EGFR inhibitors in non-small cell lung cancer. Eur. J. Med. Chem. 2018, 157, 1300–1325. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Zou, F.; Pusch, S.; Yang, L.; Zhu, Q.; Xu, Y.; Gu, Y.; von Deimling, A.; Zha, X. Design, synthesis and biological activity of 3-pyrazine-2-yl-oxazolidin-2-ones as novel, potent and selective inhibitors of mutant isocitrate dehydrogenase 1. Biorg. Med. Chem. 2017, 25, 6379–6387. [Google Scholar] [CrossRef]
- Yuan, X.; Wu, H.; Bu, H.; Zheng, P.; Zhou, J.; Zhang, H. Design, synthesis and biological evaluation of pyridone–aminal derivatives as MNK1/2 inhibitors. Biorg. Med. Chem. 2019, 27, 1211–1225. [Google Scholar] [CrossRef]
- Niculescu-Duvaz, I.; Roman, E.; Whittaker, S.R.; Friedlos, F.; Kirk, R.; Scanlon, I.J.; Davies, L.C.; Niculescu-Duvaz, D.; Marais, R.; Springer, C.J. Novel Inhibitors of the v-raf Murine Sarcoma Viral Oncogene Homologue B1 (BRAF) Based on a 2,6-Disubstituted Pyrazine Scaffold. J. Med. Chem. 2008, 51, 3261–3274. [Google Scholar] [CrossRef]
- Bregman, H.; Nguyen, H.N.; Feric, E.; Ligutti, J.; Liu, D.; McDermott, J.S.; Wilenkin, B.; Zou, A.; Huang, L.; Li, X.; et al. The discovery of aminopyrazines as novel, potent Nav1.7 antagonists: Hit-to-lead identification and SAR. Bioorg. Med. Chem. Lett. 2012, 22, 2033–2042. [Google Scholar] [CrossRef]
- Verheij, M.H.P.; Thompson, A.J.; van Muijlwijk-Koezen, J.E.; Lummis, S.C.R.; Leurs, R.; de Esch, I.J.P. Design, Synthesis, and Structure–Activity Relationships of Highly Potent 5-HT3 Receptor Ligands. J. Med. Chem. 2012, 55, 8603–8614. [Google Scholar] [CrossRef]
- Wanka, L.; Iqbal, K.; Schreiner, P.R. The Lipophilic Bullet Hits the Targets: Medicinal Chemistry of Adamantane Derivatives. Chem. Rev. 2013, 113, 3516–3604. [Google Scholar] [CrossRef] [Green Version]
- Gilligan, B.S.; Veale, J.; Wodak, J. Amantadine hydrochloride in the treatment of Parkinson’s disease. Med. J. Aust. 1970, 2, 634–637. [Google Scholar] [CrossRef]
- Hunter, K.R.; Stern, G.M.; Laurence, D.R.; Armitage, P. Amantadine in Parkinsonism. Lancet 1970, 295, 1127–1129. [Google Scholar] [CrossRef]
- Da Settimo, A.; Marini, A.M.; Primofiore, G.; Da Settimo, F. Synthesis and evaluation of aminoadamantane derivatives for in vitro anti-HIV and antitumor activities. Farmaco 1995, 50, 321–326. [Google Scholar]
- Danilenko, G.I.; Rybalko, S.L.; Maksimov, Y.N.; Baklan, V.F.; Guzhova, S.V. Adamantane-1- and norbornane-2-carboxylic acid hydrazides as HIV inhibitors. Pharm. Chem. J. 2000, 34, 23–24. [Google Scholar] [CrossRef]
- Chayrov, R.; Parisis, N.A.; Chatziathanasiadou, M.V.; Vrontaki, E.; Moschovou, K.; Melagraki, G.; Sbirkova-Dimitrova, H.; Shivachev, B.; Schmidtke, M.; Mitrev, Y.; et al. Synthetic Analogues of Aminoadamantane as Influenza Viral Inhibitors—In Vitro, in Silico and QSAR Studies. Molecules 2020, 25, 3989. [Google Scholar] [CrossRef]
- Grillaud, M.; Bianco, A. Multifunctional adamantane derivatives as new scaffolds for the multipresentation of bioactive peptides. J. Pept. Sci. 2015, 21, 330–345. [Google Scholar] [CrossRef]
- Avdyunina, N.I.; Morqzov, I.S.; Bol’shakova, R.F.; Militareva, N.A.; Klimova, N.V.; Bykov, N.P.; Pyatin, B.M.; Khranilov, A.A.; Dvalishvili, É.G. Synthesis and pharmacological properties of benzimidazoline-3-acetic acid n-adamantylamides. Pharm. Chem. J. 1988, 22, 543–546. [Google Scholar] [CrossRef]
- Baxter, A.; Bent, J.; Bowers, K.; Braddock, M.; Brough, S.; Fagura, M.; Lawson, M.; McInally, T.; Mortimore, M.; Robertson, M.; et al. Hit-to-Lead studies: The discovery of potent adamantane amide P2X7 receptor antagonists. Bioorg. Med. Chem. Lett. 2003, 13, 4047–4050. [Google Scholar] [CrossRef]
- Sorensen, B.; Rohde, J.; Wang, J.; Fung, S.; Monzon, K.; Chiou, W.; Pan, L.; Deng, X.; Stolarik, D.; Frevert, E.U.; et al. Adamantane 11-β-HSD-1 inhibitors: Application of an isocyanide multicomponent reaction. Bioorg. Med. Chem. Lett. 2006, 16, 5958–5962. [Google Scholar] [CrossRef] [PubMed]
- Rohde, J.J.; Pliushchev, M.A.; Sorensen, B.K.; Wodka, D.; Shuai, Q.; Wang, J.; Fung, S.; Monzon, K.M.; Chiou, W.J.; Pan, L.; et al. Discovery and Metabolic Stabilization of Potent and Selective 2-Amino-N-(adamant-2-yl) Acetamide 11β-Hydroxysteroid Dehydrogenase Type 1 Inhibitors. J. Med. Chem. 2007, 50, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Kim, S.; Han, H.Y.; Son, H.J.; Lee, H.J.; Shin, Y.A.; Kim, J.-S.; Park, H.-G. Synthesis and biological evaluation of picolinamides as potent inhibitors of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1). Bioorg. Med. Chem. Lett. 2015, 25, 695–700. [Google Scholar] [CrossRef]
- Collins, K.C.; Janda, K.D. Investigating Hapten Clustering as a Strategy to Enhance Vaccines against Drugs of Abuse. Bioconjugate Chem. 2014, 25, 593–600. [Google Scholar] [CrossRef]
- Stankova, I.; Chuchkov, K.; Chayrov, R.; Mukova, L.; Galabov, A.; Marinkova, D.; Danalev, D. Adamantane Derivatives Containing Thiazole Moiety: Synthesis, Antiviral and Antibacterial Activity. Int. J. Pept. Res. Ther. 2020, 26, 1781–1787. [Google Scholar] [CrossRef]
- Samson, D.; Daltrozzo, E. Synthesis of Diheteroarylamine Ligands by Palladium-Catalyzed Mono- and Diamination of Dichloroheteroarenes with Heteroarenamines. Helv. Chim. Acta 2011, 94, 46–60. [Google Scholar] [CrossRef]
- Wang, A.; Zhang, H.; Biehl, E.R. Preparation of N1-alkyl- and N1,N1-dialkylisoquinoline-1,3-diamines and 1-alkyl- and 1-phenylisoquinolin-3-amines from the reaction of α-cyano- o-tolunitrile with lithium amides, alkyllithiums, and phenyllithium. Heterocycles 2000, 53, 291–300. [Google Scholar] [CrossRef]
- Balog, J.; Riedl, Z.; Hajós, G.; Miskolczy, Z.; Biczók, L. New fluorescent isoquinoline derivatives. Tetrahedron Lett. 2011, 52, 5264–5266. [Google Scholar] [CrossRef]
- Dubovtsev, A.Y.; Shcherbakov, N.V.; Dar’in, D.V.; Kukushkin, V.Y. The Dichotomy of Gold-catalyzed Interplay between Cyanamides and Ynamides: Controllable Switch from [2+2+2] to [4+2] Cycloaddition. Adv. Synth. Catal. 2020, 362, 2672–2682. [Google Scholar] [CrossRef]
- Abel, A.S.; Grigorova, O.K.; Averin, A.D.; Maloshitskaya, O.A.; Butov, G.M.; Savelyev, E.N.; Orlinson, B.S.; Novakov, I.A.; Beletskaya, I.P. Amination of chloro-substituted heteroarenes with adamantane-containing amines. Russ. Chem. Bull. 2016, 65, 1820–1828. [Google Scholar] [CrossRef]
- Abel, A.S.; Averin, A.D.; Buryak, A.K.; Savelyev, E.N.; Orlinson, B.S.; Novakov, I.A.; Beletskaya, I.P. The Palladium-Catalyzed Heteroarylation of Adamantylalkyl Amines with Dihalogenopyridines: Scope and Limitations. Synthesis 2017, 49, 5067–5080. [Google Scholar]
- Abel, A.S.; Averin, A.D.; Maloshitskaya, O.A.; Savelyev, E.N.; Orlinson, B.S.; Novakov, I.A.; Beletskaya, I.P. Palladium-Catalyzed Amination of Dichloroquinolines with Adamantane-Containing Amines. Molecules 2013, 18, 2096–2109. [Google Scholar] [CrossRef] [Green Version]
- Abel, A.S.; Zenkov, I.S.; Averin, A.D.; Cheprakov, A.V.; Bessmertnykh-Lemeune, A.G.; Orlinson, B.S.; Beletskaya, I.P. Tuning the Luminescent Properties of Ruthenium(II) Amino-1,10-Phenanthroline Complexes by Varying the Position of the Amino Group on the Heterocycle. ChemPlusChem 2019, 84, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, J.F.; Shaughnessy, K.H.; Shekhar, S.; Green, R.A. Palladium-catalyzed amination of aryl halides. Org. React. 2019, 100, 853–958. [Google Scholar]
- Hooper, M.W.; Utsunomiya, M.; Hartwig, J.F. Scope and Mechanism of Palladium-Catalyzed Amination of Five-Membered Heterocyclic Halides. J. Org. Chem. 2003, 68, 2861–2873. [Google Scholar] [CrossRef]
- Shen, Q.; Hartwig, J.F. [(CyPF-tBu)PdCl2]: An Air-Stable, One-Component, Highly Efficient Catalyst for Amination of Heteroaryl and Aryl Halides. Org. Lett. 2008, 10, 4109–4112. [Google Scholar] [CrossRef] [Green Version]
- Lyakhovich, M.S.; Murashkina, A.V.; Averin, A.D.; Abel, A.S.; Maloshitskaya, O.A.; Savelyev, E.N.; Orlinson, B.S.; Beletskaya, I.P. Arylation of Adamantanamines: X. Palladium- and Copper-Catalyzed Heteroarylation of Adamantane-Containing Amines with Bromopyridines. Russ. J. Org. Chem. 2019, 55, 737–747. [Google Scholar] [CrossRef]
- Averin, A.D.; Ranyuk, E.R.; Golub, S.L.; Buryak, A.K.; Savelyev, E.N.; Orlinson, B.S.; Novakov, I.A.; Beletskaya, I.P. Synthesis of a New Family of Adamantylpyridin-2-amines by Palladium-Catalyzed Amination. Synthesis 2007, 2007, 2215–2221. [Google Scholar] [CrossRef]
- Grigorova, O.K.; Averin, A.D.; Abel, A.S.; Maloshitskaya, O.A.; Kovalev, V.V.; Savelev, E.N.; Orlinson, B.S.; Novakov, I.A.; Beletskaya, I.P. Arylation of adamantanamines: IV. Palladium-catalyzed arylation of amines of adamantane series with isomeric chloroquinolines. Russ. J. Org. Chem. 2012, 48, 1391–1406. [Google Scholar] [CrossRef]
- Raczyńska, E.D.; Kosińska, W.; Ośmiałowski, B.; Gawinecki, R. Tautomeric Equilibria in Relation to Pi-Electron Delocalization. Chem. Rev. 2005, 105, 3561–3612. [Google Scholar] [CrossRef]
- Raczyńska, E.D.; Kolczyńska, K.; Stępniewski, T.M. Consequences of one-electron oxidation and one-electron reduction for 4-aminopyrimidine—DFT studies. J. Mol. Model. 2012, 18, 3523–3533. [Google Scholar] [CrossRef]
- Plakhotnik, V.M.; Kovtun, V.Y.; Krasutskii, P.A.; Novikova, M.I.; Prokhorov, A.B. Derivatives of Adamantane. I. 2-Hydroxyethylamino Derivatives of Adamantane. J. Org. Chem. USSR 1981, 17, 1287–1290. [Google Scholar]
- Novakov, I.A.; Kulev, I.A.; Radchenko, S.S.; Birznieks, K.A.; Boreko, E.I.; Vladyko, G.V.; Korobchenko, L.V. Synthesis and antiviral activity of the hydrochlorides of alicyclic mono- and diamines. Pharm. Chem. J. 1987, 21, 287–291. [Google Scholar] [CrossRef]
- Novakov, I.A.; Orlinson, B.S.; Savel’ev, E.N.; Potaenkova, E.A.; Shilin, A.K. Method of Produging 2-(2-Amino) Alkyladamantanes. Patent number RU2495020C1, 10 October 2013. Available online: https://patents.google.com/patent/RU2495020C1/ru (accessed on 25 March 2021).
- Joubert, J.; Dyk, S.V.; Green, I.R.; Malan, S.F. Synthesis, evaluation and application of polycyclic fluorescent analogues as N-methyl-d-aspartate receptor and voltage gated calcium channel ligands. Eur. J. Med. Chem. 2011, 46, 5010–5020. [Google Scholar] [CrossRef]
- Ukai, T.; Kawazura, H.; Ishii, Y.; Bonnet, J.J.; Ibers, J.A. Chemistry of dibenzylideneacetone-palladium(0) complexes: I. Novel tris(dibenzylideneacetone)dipalladium(solvent) complexes and their reactions with quinones. J. Organomet. Chem. 1974, 65, 253–266. [Google Scholar] [CrossRef]








| Entry | Amine | Equiv. of Amine | Ligand | Pd(dba)2/L, mol% | Product, Yield, % |
|---|---|---|---|---|---|
| 1 | 4a | 1 | BINAP | 4/4.5 | 8a, oligomers 1 |
| 2 | 4a | 2 | Cy-JosiPhos | 2/2.5 | 8a, oligomers 1 |
| 3 | 4a | 2 | DavePhos | 4/4.5 | 8a, oligomers 1 |
| 4 | 4a | 2 | Ph-JosiPhos | 4/4.5 | 8a, oligomers 1 |
| 5 | 4a | 4 | DavePhos | 4/4.5 | 8a, 60 |
| 6 | 4a | 4 | BINAP | 8/9 | 8a, 61 |
| 7 | 4b | 4 | DavePhos | 4/4.5 | 8b, 40 |
| 8 | 4f | 4 | DavePhos | 4/4.5 | 8f, 46 |
| Entry | Compound | X | Reaction | K273 | ΔH≠, kcal/mol | ΔS≠, cal/mol × K | ΔG≠(273 K), kcal/mol |
|---|---|---|---|---|---|---|---|
| 1 | 5g | 1-CH2CH2 | A→B | 0.45 | 17.84 | 13.58 | 14.24 |
| 2 | 5g | 1-CH2CH2 | B→A | 16.89 | 12.65 | 13.81 | |
| 3 | 5b | 1-CH2 | A→B | 0.495 | 19.30 | 17.51 | 14.67 |
| 4 | 5b | 1-CH2 | B→A | 18.46 | 15.81 | 14.29 | |
| 5 | 5c | 2-CH2 | A→B | 0.54 | 16.31 | 7.38 | 14.48 |
| 6 | 5c | 2-CH2 | B→A | 15.42 | 5.30 | 14.15 | |
| 7 | 5f | 1-CH2CH(CH3) | A→B | 0.575 | 17.36 | 11.90 | 14.26 |
| 8 | 5f | 1-CH2CH(CH3) | B→A | 16.69 | 10.59 | 13.96 |
| Entry | Amine | Equiv. of Amine | Ligand | Product, Yield (%) |
|---|---|---|---|---|
| 1 | 4a | 1 | BINAP | 9a, oligomers a |
| 2 | 4a | 1 | DavePhos | 9a, oligomers a |
| 3 | 4a | 2 | Cy-JosiPhos | 9a, 30 |
| 4 | 4a | 4 | Ph-JosiPhos | 9a, 90 |
| 5 | 4b | 4 | Ph-JosiPhos | 9b, 42 |
| 6 | 4f | 4 | Ph-JosiPhos | 9f, (47) b |
| 7 | 4g | 4 | Ph-JosiPhos | 9g, 48 |
| 8 | 4h | 4 | Ph-JosiPhos | 9h, 36 |
| Entry | Amine | Equiv. of Amine | Ligand | Pd(dba)2/L, mol% | Product, Yield |
|---|---|---|---|---|---|
| 1 | 4a | 2 | Cy-JosiPhos | 4/4.5 | 10a, 11a |
| 2 | 4a | 2 | DavePhos | 4/4.5 | 10a, oligomers a |
| 3 | 4a | 4 | DavePhos | 4/4.5 | 10a, 77% |
| 4 | 4a | 4 | BINAP | 8/9 | 10a, 76% |
| 5 | 4b | 4 | DavePhos | 4/4.5 | 10b, 62% |
| 6 | 4f | 4 | DavePhos | 4/4.5 | 10f, 67% |
| 7 | 4g | 4 | DavePhos | 4/4.5 | 10g, 43% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharlamova, A.D.; Abel, A.S.; Averin, A.D.; Maloshitskaya, O.A.; Roznyatovskiy, V.A.; Savelyev, E.N.; Orlinson, B.S.; Novakov, I.A.; Beletskaya, I.P. Mono- and Diamination of 4,6-Dichloropyrimidine, 2,6-Dichloropyrazine and 1,3-Dichloroisoquinoline with Adamantane-Containing Amines. Molecules 2021, 26, 1910. https://doi.org/10.3390/molecules26071910
Kharlamova AD, Abel AS, Averin AD, Maloshitskaya OA, Roznyatovskiy VA, Savelyev EN, Orlinson BS, Novakov IA, Beletskaya IP. Mono- and Diamination of 4,6-Dichloropyrimidine, 2,6-Dichloropyrazine and 1,3-Dichloroisoquinoline with Adamantane-Containing Amines. Molecules. 2021; 26(7):1910. https://doi.org/10.3390/molecules26071910
Chicago/Turabian StyleKharlamova, Alisa D., Anton S. Abel, Alexei D. Averin, Olga A. Maloshitskaya, Vitaly A. Roznyatovskiy, Evgenii N. Savelyev, Boris S. Orlinson, Ivan A. Novakov, and Irina P. Beletskaya. 2021. "Mono- and Diamination of 4,6-Dichloropyrimidine, 2,6-Dichloropyrazine and 1,3-Dichloroisoquinoline with Adamantane-Containing Amines" Molecules 26, no. 7: 1910. https://doi.org/10.3390/molecules26071910
APA StyleKharlamova, A. D., Abel, A. S., Averin, A. D., Maloshitskaya, O. A., Roznyatovskiy, V. A., Savelyev, E. N., Orlinson, B. S., Novakov, I. A., & Beletskaya, I. P. (2021). Mono- and Diamination of 4,6-Dichloropyrimidine, 2,6-Dichloropyrazine and 1,3-Dichloroisoquinoline with Adamantane-Containing Amines. Molecules, 26(7), 1910. https://doi.org/10.3390/molecules26071910