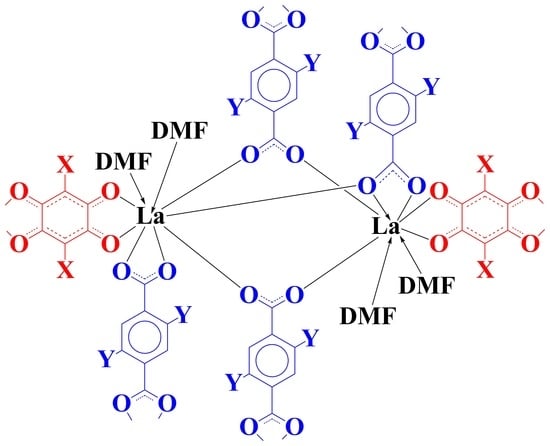
Heteroleptic LaIII Anilate/Dicarboxylate Based Neutral 3D-Coordination Polymers
Abstract
:1. Introduction
2. Results and Discussion
- 1:
- (A) −x + 1, −y + 1, −z + 1; (B) −x + 1, −y + 1, −z; (C) −x, −y + 2, −z + 1; (D) −x + 2, −y + 1, −z + 1
- 2:
- (A) −x + 1, −y + 1, −z + 1; (B) −x + 1, −y + 1, −z; (C) −x + 1, −y, −z + 1; (D) −x, −y + 1, −z + 1
- 3:
- (A) −x + 3, −y, −z + 2; (B) −x + 5/2, y + 1/2, −z + 3/2; (C) x + 1/2, −y−1/2, z + 1/2; (D) −x + 2, −y, −z + 2; (E) x + 1/2, −y −1/2, z + 1/2
- 4:
- (A) −x + 1, −y + 2, −z + 2; (B) −x + 1, −y + 1, −z + 2; (C) −x, −y + 1, −z + 1.
3. Materials and Methods
3.1. Reagents and Methods
3.2. Synthesis
3.2.1. Synthesis of 1
3.2.2. Synthesis of 2
3.2.3. Synthesis of 3
3.2.4. Synthesis of 4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lysova, A.A.; Samsonenko, D.G.; Dorovatovskii, P.V.; Lazarenko, V.A.; Khrustalev, V.N.; Kovalenko, K.A.; Dybtsev, D.N.; Fedin, V.P. Tuning the Molecular and Cationic Affinity in a Series of Multifunctional Metal−Organic Frameworks Based on Dodecanuclear Zn(II) Carboxylate Wheels. J. Am. Chem. Soc. 2019, 141, 17260–17269. [Google Scholar] [CrossRef]
- Lysova, A.A.; Samsonenko, D.G.; Kovalenko, K.A.; Nizovtsev, A.S.; Dybtsev, D.N.; Fedin, V.P. A Series of Mesoporous Metal-Organic Frameworks with Tunable Windows Sizes and Exceptionally High Ethane over Ethylene Adsorption Selectivity. Angew. Chem. Int. Ed. 2020, 59, 20561–20567. [Google Scholar] [CrossRef]
- Adil, K.; Belmabkhout, Y.; Pillai, R.S.; Cadiau, A.; Bhatt, P.M.; Assen, A.H.; Maurinb, G.; Eddaoudi, M. Gas/vapour separation using ultra-microporous metal–organic frameworks: Insights into the structure/separation relationship. Chem. Soc. Rev. 2017, 46, 3402–3430. [Google Scholar] [CrossRef]
- Yao, X.; Wang, X.; Han, Y.; Yan, P.; Li, Y.; Li, G. Structure, color-tunable luminescence, and UV-vis/NIR benzaldehyde detection of lanthanide coordination polymers based on two fluorinated ligands. CrystEngComm 2018, 20, 3335–3343. [Google Scholar] [CrossRef]
- Ren, Y.-X.; Zhao, X.-L.; Wang, Z.-X.; Pan, Y.; Li, H.-P.; Wang, F.-Y.; Zhu, S.-F.; Shao, C.-H. Effects of template molecules on the structures and luminescence intensities of a series of porous Tb-MOFs based on the 2-nitroterephthalate ligand. RSC Adv. 2018, 8, 17497–17503. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhan, Z.; Liang, X.; Chen, C.; Liu, X.; Jia, Y.; Hu, M. Lanthanide-MOFs constructed from mixed dicarboxylate ligands as selective multi-responsive luminescent sensors. Dalton Trans. 2018, 47, 3272–3282. [Google Scholar] [CrossRef] [PubMed]
- Sahadevan, S.A.; Monni, N.; Oggianu, M.; Abhervé, A.; Marongiu, D.; Saba, M.; Mura, A.; Bongiovanni, G.; Mameli, V.; Cannas, C.; et al. Heteroleptic NIR-Emitting YbIII/Anilate-Based Neutral Coordination Polymer Nanosheets for Solvent Sensing. ACS Appl. Nano Mater. 2020, 3, 94–104. [Google Scholar] [CrossRef] [Green Version]
- D’Alessandro, D.M. Exploiting redox activity in metal–organic frameworks: Concepts, trends and perspectives. Chem. Commun. 2016, 52, 8957–8971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; DeGayner, J.A.; Sun, L.; Zee, D.Z.; Harris, T.D. Reversible redox switching of magnetic order and electrical conductivity in a 2D manganese benzoquinoid framework. Chem. Sci. 2019, 10, 4652–4661. [Google Scholar] [CrossRef] [Green Version]
- Burnett, D.L.; Oozeerally, R.; Pertiwi, R.; Chamberlain, T.W.; Cherkasov, N.; Clarkson, G.J.; Krisnandi, Y.K.; Degirmenci, V.; Walton, R.I. A hydrothermally stable ytterbium metal–organic framework as a bifunctional solid-acid catalyst for glucose conversion. Chem. Commun. 2019, 55, 11446–11449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Zhao, D. Metal–organic frameworks with Lewis acidity: Synthesis, characterization, and catalytic applications. CrystEngComm 2017, 19, 4066–4081. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.-X.; Gu, X.-M.; Zhang, W.-L.; Ni, L. Structure Variation from One-Dimensional Chain to Three-Dimensional Architecture: Effect of Ligand on Construction of Lanthanide Coordination Polymers. J. Chem. Sci. 2017, 129, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Wen, H.-M.; Cui, Y.; Zhou, W.; Qian, G.; Chen, B. Emerging Multifunctional Metal–Organic Framework Materials. Adv. Mater. 2016, 28, 8819–8860. [Google Scholar] [CrossRef]
- Chen, G.; Gee, L.B.; Xu, W.; Zhu, Y.; Lezama-Pacheco, J.S.; Huang, Z.; Li, Z.; Babicz, J.T.; Choudhury, J.S.; Chang, T.-H.; et al. Valence-Dependent Electrical Conductivity in a 3D Tetrahydroxyquinone-Based Metal−Organic Framework. J. Am. Chem. Soc. 2020, 142, 21243–21248. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, M.L.; Congiu, F.; Concas, G.; Sahadevan, S.A. Recent Advances on Anilato-Based Molecular Materials with Magnetic and/or Conducting Properties. Magnetochemistry 2017, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Sekine, Y.; Komatsumaru, Y.; Hayami, S.; Miyasaka, H. Thermally Induced Valence Tautomeric Transition in a Two-Dimensional Fe-Tetraoxolene Honeycomb Network. Angew. Chem. Int. Ed. 2018, 57, 12043–12047. [Google Scholar] [CrossRef]
- Martínez-Hernández, C.; Gómez-Claramunt, P.; Benmansour, S.; Gómez-García, C.J. Pre- and post-syntheticmodulation of the ordering temperatures in a family of anilato-based magnets. Dalton Trans. 2019, 48, 13212–13223. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Kawata, S. Coordination compounds of 1,4-dihydroxybenzoquinone and its homologues. Structures and properties. Coord. Chemi. Rev. 2002, 224, 11–34. [Google Scholar] [CrossRef]
- Bondaruk, K.; Hua, C. Effect of Counterions on the Formation and Structures of Ce(III) and Er(III) Chloranilate Frameworks. Cryst. Growth Des. 2019, 19, 3338–3347. [Google Scholar] [CrossRef]
- AndrosˇDubraja, L.; Molcanov, K.; Zilic, D.; Kojic-Prodic, B.; Wenger, E. Multifunctionality and size of the chloranilate ligand define the topology of transition metal coordination polymers. New J. Chem. 2017, 41, 6785–6794. [Google Scholar] [CrossRef]
- Martínez-Hernández, C.; Benmansour, S.; García, C.J.G. Modulation of the ordering temperature in anilato-based magnets. Polyhedron 2019, 170, 122–131. [Google Scholar] [CrossRef]
- Hernández-Paredes, A.; Cerezo-Navarrete, C.; García, C.J.G.; Benmansour, S. Slow relaxation in doped coordination polymers and dimers based on lanthanoids and anilato ligands. Polyhedron 2019, 170, 476–485. [Google Scholar] [CrossRef]
- Murase, R.; Abrahams, B.F.; D’Alessandro, D.M.; Davies, C.G.; Hudson, T.A.; Jameson, G.N.L.; Moubaraki, B.; Murray, K.S.; Robson, R.; Sutton, A.L. Mixed Valency in a 3D Semiconducting Iron−Fluoranilate Coordination Polymer. Inorg. Chem. 2017, 56, 9025–9035. [Google Scholar] [CrossRef] [PubMed]
- Kingsbury, C.J.; Abrahams, B.F.; D’Alessandro, D.M.; Hudson, T.A.; Murase, R.; Robson, R.; White, K.F. Role of NEt4+ in Orienting and Locking Together [M2lig3]2− (6,3) Sheets (H2lig = Chloranilic or Fluoranilic Acid) to Generate Spacious Channels Perpendicular to the Sheets. Cryst. Growth Des. 2017, 17, 1465–1470. [Google Scholar] [CrossRef]
- Simonson, A.N.; Kareis, C.M.; Ovanesyan, N.S.; Baumann, D.O.; Rheingold, A.L.; Arif, A.M.; Miller, J.S. Seven-coordinate tetraoxolate complexes. Polyhedron 2018, 139, 215–221. [Google Scholar] [CrossRef]
- Benmansour, S.; Gómez-García, C.J. A Heterobimetallic Anionic 3,6-Connected 2D Coordination Polymer Based on Nitranilate as Ligand. Polymers 2016, 8, 89. [Google Scholar] [CrossRef] [Green Version]
- Kingsbury, C.J.; Abrahams, B.F.; Auckett, J.E.; Chevreau, H.; Dharma, A.D.; Duyker, S.; He, Q.; Hua, C.; Hudson, T.A.; Murray, K.S.; et al. Square Grid Metal–Chloranilate Networks as Robust HostSystems for GuestSorption. Chem. Eur. J. 2019, 25, 5222–5234. [Google Scholar] [CrossRef]
- Gómez-Claramunt, P.; Benmansour, S.; Hernández-Paredes, A.; Cerezo-Navarrete, C.; Rodríguez-Fernández, C.; Canet-Ferrer, J.; Cantarero, A.; Gómez-García, C.J. Tuning the Structure and Properties of Lanthanoid Coordination Polymers with an Asymmetric Anilato Ligand. Magnetochemistry 2018, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- Kharitonov, A.D.; Trofimova, O.Y.; Meshcheryakova, I.N.; Fukin, G.K.; Khrizanforov, M.N.; Budnikova, Y.H.; Bogomyakov, A.S.; Aysin, R.R.; Kovalenko, K.A.; Piskunov, A.V. 2D-Metal-organic coordination polymers of lanthanides (La(III), Pr(III) and Nd(III)) with redox-active dioxolene bridging ligand. CrystEngComm 2020, 22, 4675–4679. [Google Scholar] [CrossRef]
- Abrahams, B.F.; Coleiro, J.; Ha, K.; Hoskins, B.F.; Orchard, S.D.; Robson, R. Dihydroxybenzoquinone and chloranilic acid derivatives of rare earth metals. Dalton Trans. 2002, 8, 1586–1594. [Google Scholar] [CrossRef]
- Luo, J.; Liu, B.S.; Cao, C.; Wei, F. Neodymium(III) organic frameworks (Nd-MOF) as near infrared fluorescent probe for highly selectively sensing of Cu2+. Inorg. Chem. Comm. 2017, 76, 18–21. [Google Scholar] [CrossRef]
- Li, C.Y.; Cai, D.M.; Yin, J.C.; Cai, L.P.; Zeng, M.; Wang, J.; Zhu, W.H. Crystal Structure, Fluorescence Spectroscopy, and Electrochemical Property of Two Neodymium Coordination Polymers with Phenoxy Acids. Russ. J. Coord. Chem. 2016, 42, 476–485. [Google Scholar] [CrossRef]
- Benmansour, S.; Gómez-García, C.J. Lanthanoid-Anilato Complexes and Lattices. Magnetochemistry 2020, 6, 71. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Li, X.; Zhai, F.; Yan, S.; Liu, N.; Chai, Z.; Xu, Y.; Ouyang, X.; Wang, S. Direct Radiation Detection by a Semiconductive Metal−Organic Framework. J. Am. Chem. Soc. 2019, 141, 8030–8034. [Google Scholar] [CrossRef]
- Artizzu, F.; Atzori, M.; Liu, J.; Mara, D.; Hecke, K.V.; Deun, R.V. Solution-processable Yb/Er 2D-layered metallorganic frameworks with high NIR-emission quantum yields. J. Mater. Chem. C 2019, 7, 11207–11214. [Google Scholar] [CrossRef]
- Chang, C.-H.; Li, A.-C.; Popovs, I.; Kaveevivitchai, W.; Chen, J.-L.; Chou, K.-C.; Kuof, T.-S.; Chen, T.-H. Elucidating metal and ligand redox activities of a copper-benzoquinoid coordination polymer as the cathode for lithium-ion batteries. J. Mater. Chem. A 2019, 7, 23770–23774. [Google Scholar] [CrossRef]
- Alexandrov, E.V.; Blatov, V.A.; Kochetkov, A.V.; Proserpio, D.M. Underlying nets in three-periodic coordination polymers: Topology, taxonomy and prediction from a computer-aided analysis of the Cambridge Structural Database. CrystEngComm 2011, 13, 3947–3958. [Google Scholar] [CrossRef]
- Alvarez, S.; Alemany, P.; Casanova, D.; Cirera, J.; Llunell, M.; Avnir, D. Shape maps and polyhedral interconversion paths in transition metal chemistry. Coord. Chem. Rev. 2005, 249, 1693–1708. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE (2.1); Universitat de Barcelona: Barcelona, Spain, 2013. [Google Scholar]
- Ruiz-Martinez, A.; Casanova, D.; Alvarez, S. Polyhedral Structures with an Odd Number ofVertices: Nine-Coordinate Metal Compounds. Chem. Eur. J. 2008, 14, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Milašinović, V.; Molčanov, K. Nitranilic acid as a basis for construction of coordination polymers: From discrete monomers to 3D networks. CrystEngComm 2019, 21, 2962–2969. [Google Scholar] [CrossRef]
- Vuković, V.; Molcanov, K.I.; Jelsch, C.; Wenger, E.; Krawczuk, A.; Jurić, M.; Dubraja, L.A.; Kojić-Prodić, B. Malleable Electronic Structure of Chloranilic Acid and Its Species Determined by X-ray Charge Density Studies. Cryst. Growth Des. 2019, 19, 2802–2810. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Li, L.; Ma, C.; Shen, Z.; Song, Y.; You, X. Structures and Properties of Porous Coordination Polymers Based on Lanthanide Carboxylate Building Units. Inorg. Chem. 2010, 49, 10781–10787. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Jiang, Y.-L.; Xiahou, Z.-J.; Fu, J.-H.; Liu, Q.-Y. Diversity of lanthanide(III)-2,5-dihydroxy-1,4-benzenedicarboxylate extended frameworks: Syntheses, structures, and magnetic properties. Dalton Trans. 2012, 41, 11428–11437. [Google Scholar] [CrossRef] [PubMed]
- Barbour, L.J. Crystal porosity and the burden of proof. Chem. Commun. 2006, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Khamaletdinova, N.M.; Meshcheryakova, I.N.; Piskunov, A.V.; Kuznetsova, O.V. Experimental and theoretical study of the vibrational spectra of tin(IV) complexes based on 2-hydroxy-3,6-di-tert-butyl-para-benzoquianone. J. Struct. Chem. 2015, 56, 233–242. [Google Scholar] [CrossRef]
- Perrin, D.D.; Armarego, W.L.F.; Perrin, D.R. Purification of Laboratory Chemicals; Pergamon: Oxford, UK, 1980. [Google Scholar]
- Bruker. APEX3; Bruker AXS Inc.: Madison, WI, USA, 2018. [Google Scholar]
- Rigaku Oxford Diffraction; CrysAlisPro Ver. 1.171.37.35; Rigaku Corporation: Wroclaw, Poland, 2014.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystalstructure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Cryst. C 2015, 71, 9–18. [Google Scholar] [CrossRef] [Green Version]














| Bond | 1 | Bond | 2∙2DMF |
| La(1)-O(1) | 2.477(2) | La(1)-O(1) | 2.460(2) |
| La(1)-O(2B) | 2.516(2) | La(1)-O(2B) | 2.505(2) |
| La(1)-O(3) | 2.513(2) | La(1)-O(3) | 2.495(2) |
| La(1)-O(4A) | 2.510(2) | La(1)-O(4A) | 2.48(2) |
| La(1)-O(5) | 2.582(2) | La(1)-O(6) | 2.514(2) |
| La(1)-O(5A) | 2.731(2) | La(1)-O(6A) | 2.849(2) |
| La(1)-O(6A) | 2.608(2) | La(1)-O(7A) | 2.518(5) |
| La(1)-O(7) | 2.568(2) | La(1)-O(9) | 2.569(2) |
| La(1)-O(8) | 2.580(5) | La(1)-O(10) | 2.496(6) |
| O(1)-C(1) | 1.271(2) | O(1)-C(1) | 1.267(2) |
| O(2)-C(3) | 1.267(2) | O(2)-C(3) | 1.259(2) |
| C(1)-C(2) | 1.409(3) | C(1)-C(2) | 1.402(3) |
| C(1)-C(3B) | 1.552(3) | C(1)-C(3B) | 1.540(3) |
| C(2)-C(3) | 1.404(3) | C(2)-C(3) | 1.411(3) |
| O(3)-C(8) | 1.262(2) | O(3)-C(8) | 1.263(4) |
| O(4)-C(8) | 1.263(2) | O(4)-C(8) | 1.319(5) |
| C(8)-C(9) | 1.511(2) | C(8)-C(9) | 1.499(4) |
| C(9)-C(10) | 1.388(3) | C(9)-C(10) | 1.390(4) |
| C(9)-C(11C) | 1.390(3) | C(9)-C(11C) | 1.35(2) |
| C(10)-C(11) | 1.389(3) | C(10)-C(11) | 1.393(5) |
| O(5)-C(12) | 1.245(2) | O(6)-C(12) | 1.275(3) |
| O(6)-C(12) | 1.255(2) | O(7)-C(12) | 1.261(4) |
| C(12)-C(13) | 1.501(3) | C(12)-C(13) | 1.486(4) |
| C(13)-C(15D) | 1.396(3) | C(13)-C(14) | 1.407(4) |
| C(13)-C(14) | 1.398(3) | C(15)-C(13) | 1.391(4) |
| C(14)-C(15) | 1.390(3) | C(15)-C(14D) | 1.654(6) |
| Bond | 3∙0.6DMF | Bond | 4 |
| La(1)-O(1) | 2.58(2) | La(1)-O(1) | 2.463(7) |
| La(1)-O(2D) | 2.572(7) | La(1)-O(2A) | 2.458(7) |
| La(1)-O(3) | 2.474(3) | La(1)-O(3) | 2.488(6) |
| La(1)-O(4A) | 2.466(3) | La(1)-O(4C) | 2.503(6) |
| La(1)-O(5C) | 2.567(3) | La(1)-O(5) | 2.488(6) |
| La(1)-O(6B) | 2.501(3) | La(1)-O(6B) | 2.495(6) |
| La(1)-O(6E) | 2.729(3) | La(1)-O(7) | 2.52(2) |
| La(1)-O(7) | 2.511(6) | La(1)-O(8) | 2.53(2) |
| La(1)-O(8) | 2.494(7) | O(1)-C(1) | 1.29(2) |
| O(1)-C(1) | 1.253(6) | O(2)-C(3) | 1.28(2) |
| O(2)-C(3) | 1.251(6) | C(1)-C(2) | 1.378(7) |
| C(1)-C(2) | 1.398(7) | C(1)-C(3A) | 1.54(2) |
| C(1)-C(3D) | 1.58(2) | C(2)-C(3) | 1.377(7) |
| C(2)-C(3) | 1.414(7) | O(3)-C(8) | 1.288(9) |
| O(3)-C(4) | 1.256(6) | O(4)-C(10) | 1.28(2) |
| O(4)-C(4) | 1.260(6) | C(8)-C(9) | 1.365(7) |
| O(5)-C(11) | 1.252(6) | C(8)-C(10C) | 1.550(5) |
| O(6)-C(11) | 1.261(5) | C(9)-C(10) | 1.378(7) |
| C(4)-C(5) | 1.508(6) | O(5)-C(15) | 1.32(2) |
| C(5)-C(6) | 1.386(7) | O(6)-C(17) | 1.28(2) |
| C(5)-C(10) | 1.391(7) | C(15)-C(16) | 1.393(7) |
| C(6)-C(7) | 1.393(7) | C(15)-C(17B) | 1.551(5) |
| C(7)-C(8) | 1.392(6) | C(16)-C(17) | 1.376(7) |
| C(8)-C(9) | 1.391(7) | ||
| C(9)-C(10) | 1.389(7) | ||
| C(8)-C(11) | 1.497(6) |
| Activation | Specific Surface Area/m2·g−1 | Vpore/cm3·g−1 | Vads(CO2) a/cm3(STP)·g−1 | |||
|---|---|---|---|---|---|---|
| Langmuir | BET | DFT | Total a | DFT | ||
| 2 h | 237.3 | 111.2 | 61.6 | 0.0859 | 0.0604 | 78.9 |
| 6 h | 145.3 | 91.3 | 86.1 | 0.0727 | 0.0440 | 34.3 |
| Compound | 1 | 2∙2DMF | 3∙0.6DMF | 4 |
|---|---|---|---|---|
| Formula | C42H54La2N4O16 | C48H68La2N6O22 | C35.80H40.20Cl2La2N4.60 O16.60 | C54H82La2N4O16 |
| Formula weight | 1148.71 | 1358.90 | 1149.24 | 1321.06 |
| Crystal system | Triclinic | Triclinic | Monoclinic | Triclinic |
| Space group | P-1 | P-1 | P21/n | P-1 |
| a, Å | 10.1266(10) | 10.6159(4) | 12.4139(5) | 10.471(2) |
| b, Å | 10.3128(10) | 11.4493(4) | 12.9185(5) | 13.1311(17) |
| c, Å | 12.3495(12) | 12.4139(4) | 14.7089(6) | 13.8254(18) |
| α, deg | 81.021(3) | 90.5437(13) | 90 | 112.436(12) |
| β, deg | 75.024(3) | 108.8847(13) | 103.7170(11) | 92.458(16) |
| γ, deg | 68.767(3) | 92.3540(13) | 90 | 108.076(16) |
| V, A3 | 1158.5(2) | 1426.01(9) | 2291.57(16) | 1642.0(5) |
| Z | 1 | 1 | 2 | 1 |
| dcalc, g/cm3 | 1.646 | 1.582 | 1.666 | 1.336 |
| θ range, ° | 2.48–28.74 | 2.03–35.63 | 2.46–30.00 | 2.98–26.60 |
| Crystal size, mm | 0.20 × 0.05 × 0.05 | 0.38 × 0.33 × 0.08 | 0.20 × 0.10 × 0.06 | 0.25 × 0.14 × 0.05 |
| μ, mm−1 | 1.892 | 1.559 | 2.027 | 1.344 |
| Reflnscollected/unique | 18,483/5986 | 28,659/13,079 | 31,260/6682 | 34,062/20,437 |
| Unique reflns [I > 2σ(I)] | 5683 | 11,716 | 5298 | 10,195 |
| Rint | 0.0193 | 0.0242 | 0.0538 | 0.0755 |
| S(F2) | 1.057 | 1.056 | 1.062 | 1.048 |
| R1, wR2 [I > 2σ(I)] | 0.0198, 0.0464 | 0.0334, 0.0755 | 0.0489, 0.1144 | 0.0731, 0.1726 |
| R1, wR2 (all data) | 0.0220, 0.0471 | 0.0399, 0.0780 | 0.0696, 0.1213 | 0.1361, 0.1916 |
| Δρmax/Δρmin, e/Å3 | 1.35/−0.61 | 1.84/−1.21 | 2.16/−1.49 | 1.72/−1.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trofimova, O.Y.; Maleeva, A.V.; Ershova, I.V.; Cherkasov, A.V.; Fukin, G.K.; Aysin, R.R.; Kovalenko, K.A.; Piskunov, A.V. Heteroleptic LaIII Anilate/Dicarboxylate Based Neutral 3D-Coordination Polymers. Molecules 2021, 26, 2486. https://doi.org/10.3390/molecules26092486
Trofimova OY, Maleeva AV, Ershova IV, Cherkasov AV, Fukin GK, Aysin RR, Kovalenko KA, Piskunov AV. Heteroleptic LaIII Anilate/Dicarboxylate Based Neutral 3D-Coordination Polymers. Molecules. 2021; 26(9):2486. https://doi.org/10.3390/molecules26092486
Chicago/Turabian StyleTrofimova, Olesya Y., Arina V. Maleeva, Irina V. Ershova, Anton V. Cherkasov, Georgy K. Fukin, Rinat R. Aysin, Konstantin A. Kovalenko, and Alexandr V. Piskunov. 2021. "Heteroleptic LaIII Anilate/Dicarboxylate Based Neutral 3D-Coordination Polymers" Molecules 26, no. 9: 2486. https://doi.org/10.3390/molecules26092486
APA StyleTrofimova, O. Y., Maleeva, A. V., Ershova, I. V., Cherkasov, A. V., Fukin, G. K., Aysin, R. R., Kovalenko, K. A., & Piskunov, A. V. (2021). Heteroleptic LaIII Anilate/Dicarboxylate Based Neutral 3D-Coordination Polymers. Molecules, 26(9), 2486. https://doi.org/10.3390/molecules26092486