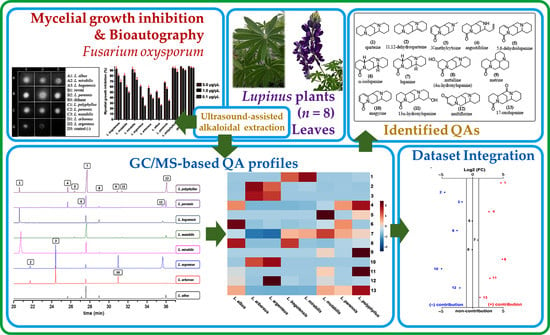
Quinolizidine-Based Variations and Antifungal Activity of Eight Lupinus Species Grown under Greenhouse Conditions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characteristics of Propagated Plants
2.2. Chemical Analysis
2.3. Direct Bioautography Assay
2.4. Mycelial Growth Inhibition Assay
2.5. Variation in the QA Profiles of L. polyphyllus Induced by Pruning Events
3. Materials and Methods
3.1. Propagation of Lupinus Plants under Greenhouse Conditions
3.2. Preparation of Quinolizidine-Rich Extracts (QREs)
3.3. Gas Chromatography Coupled to Mass Spectrometry (GC-MS)
3.4. Direct Bioautography Assay
3.5. Mycelial Growth Inhibition Assay
3.6. Fungicidal (FC) and Fungistatic (FS) Activity
3.7. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Krishna, P.M.; Rao, K.N.V.; Sandhya, S.; Banji, D. A review on phytochemical, ethnomedical and pharmacological studies on genus Sophora, Fabaceae. Braz. J. Pharmacogn. 2012, 22, 1145–1154. [Google Scholar] [CrossRef] [Green Version]
- Wink, M. Evolution of secondary metabolites in legumes (Fabaceae). S. Afr. J. Bot. 2013, 89, 164–175. [Google Scholar] [CrossRef] [Green Version]
- Confortin, T.C.; Todero, I.; Soares, J.F.; Brun, T.; Luft, L.; Ugalde, G.A.; Prá, V.D.; Mazutti, M.A.; Zabot, G.L.; Tres, M.V. Extraction and composition of extracts obtained from Lupinus albescens using supercritical carbon dioxide and compressed liquefied petroleum gas. J. Supercrit. Fluids 2017, 128, 395–403. [Google Scholar] [CrossRef]
- Kole, C. Wild Crop Relatives: Genomic and Breeding Resources. Legume Crops and Forages; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 9783642143878. [Google Scholar]
- Gresta, F.; Wink, M.; Prins, U.; Abberton, M.; Capraro, J.; Scarafoni, A.; Hill, G. Lupins in European Cropping Systems. In Legumes in Cropping Systems; Murphy-Bokern, D., Stoddard, F., Watson, C., Eds.; CABI Publishing: Wallingford, CT, USA, 2017; pp. 88–108. [Google Scholar]
- Contreras-Ortiz, N.; Jara-Muñoz, O.A.; Hughes, C.E. The acaulescent rosette species of Lupinus L. (Fabaceae) of Colombia and Ecuador including a new species from Colombia. Phytotaxa 2018, 364, 61–70. [Google Scholar] [CrossRef]
- Lucas, M.M.; Stoddard, F.; Annicchiarico, P.; Frias, J.; Martinez-Villaluenga, C.; Sussmann, D.; Duranti, M.; Seger, A.; Zander, P.; Pueyo, J. The future of lupin as a protein crop in Europe. Front. Plant Sci. 2015, 6, 705. [Google Scholar] [CrossRef]
- Cortés-Avendaño, P.; Tarvainen, M.; Suomela, J.P.; Glorio-Paulet, P.; Yang, B.; Repo-Carrasco-Valencia, R. Profile and content of residual alkaloids in ten ecotypes of Lupinus mutabilis Sweet after aqueous debittering process. Plant Foods Hum. Nutr. 2020, 75, 184–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wink, M. Introduction: Biochemistry, Physiology and Ecological Functions of Secondary Metabolites. In Biochemistry of Plant Secondary Metabolism; Wiley-Blackwell: Oxford, UK, 2010; Volume 40, pp. 1–19. ISBN 9781444320503. [Google Scholar]
- Hernández, E.M.; Rangel, M.L.C.; Corona, A.E.; del Angel, J.A.C.; López, J.A.S.; Sporer, F.; Wink, M.; Torres, K.B. Quinolizidine alkaloid composition in different organs of Lupinus aschenbornii. Rev. Bras. Farmacogn. 2011, 21, 824–828. [Google Scholar] [CrossRef] [Green Version]
- Neto, A.T.; Oliveira, C.Q.; Ilha, V.; Pedroso, M.; Burrow, R.A.; Dalcol, I.I.; Morel, A.F. Quinolizidine alkaloids from Lupinus lanatus. J. Mol. Struct. 2011, 1004, 174–177. [Google Scholar] [CrossRef]
- Aisyah, S.; Vincken, J.P.; Andini, S.; Mardiah, Z.; Gruppen, H. Compositional changes in (iso)flavonoids and estrogenic activity of three edible Lupinus species by germination and Rhizopus-elicitation. Phytochemistry 2016, 122, 65–75. [Google Scholar] [CrossRef]
- Frick, K.M.; Kamphuis, L.G.; Siddique, K.H.M.; Singh, K.B.; Foley, R.C. Quinolizidine alkaloid biosynthesis in Lupins and prospects for grain quality improvement. Front. Plant Sci. 2017, 8, 87. [Google Scholar] [CrossRef]
- Boschin, G.; Resta, D. Alkaloids Derived from Lysine: Quinolizidine (a focus on Lupin alkaloids) BT—Natural products: Phytochemistry, botany and metabolism of alkaloids, phenolics and terpenes. In Natural Products. Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 381–403. ISBN 978-3-642-22144-6. [Google Scholar]
- Wink, M. Plant secondary metabolites modulate insect behavior-steps toward addiction? Front. Physiol. 2018, 9, 364. [Google Scholar] [CrossRef] [Green Version]
- Arias Alemán, L.S.E.; Ulloa Ramones, L.A.; Rojas Oviedo, L.A.; Noboa Abdo, T.E. Effect of alkaloids of Lupinus mutabilis Sweet on gastrointestinal parasites in guinea pigs. Cienc. Digit. 2019, 3, 221–228. [Google Scholar] [CrossRef]
- Wink, M. Chemical defense of Lupins. Mollusc-repellent properties of Quinolizidine Alkaloids. Z. Naturforsch. C 1984, 39, 553–558. [Google Scholar] [CrossRef]
- Romeo, F.; Fabroni, S.; Ballistreri, G.; Muccilli, S.; Spina, A.; Rapisarda, P. Characterization and antimicrobial activity of alkaloid extracts from seeds of different genotypes of Lupinus spp. Sustainability 2018, 10, 788. [Google Scholar] [CrossRef] [Green Version]
- Ohadoma, S.C.; Nnatuanya, I.; Amazu, L.U.; Okolo, C.E. Antimicrobial activity of the leaf extract and fractions of Lupinus arboreus. J. Med. Plants Res. 2014, 8, 386–391. [Google Scholar] [CrossRef] [Green Version]
- Confortin, T.C.; Todero, I.; Soares, J.F.; Luft, L.; Brun, T.; Rabuske, J.E.; Nogueira, C.U.; Mazutti, M.A.; Zabot, G.L.; Tres, M.V. Extracts from Lupinus albescens: Antioxidant power and antifungal activity in vitro against phytopathogenic fungi. Environ. Technol. 2019, 40, 1668–1675. [Google Scholar] [CrossRef] [PubMed]
- Erdemoglu, N.; Ozkan, S.; Tosun, F. Alkaloid profile and antimicrobial activity of Lupinus angustifolius L. alkaloid extract. Phytochem. Rev. 2007, 6, 197–201. [Google Scholar] [CrossRef]
- El-Shazly, A.; Ateya, A.M.; Wink, M. Quinolizidine alkaloids profiles of Lupinus varius orientalis, L. albus albus, L. hartweguii and L. desinflorus. Z. Naturforsch. C 2001, 56, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Alcocer, A.; Zamora-Natera, J.F.; Virgen-calleros, G.; Nuño-romero, R. In vitro biological activity of extracts of Lupinus spp. on phytopathogenic fungi. Rev. Mex. Fitopatol. 2005, 23, 140–146. [Google Scholar]
- Pegg, K.G.; Coates, L.M.; O’Neill, W.T.; Turner, D.W. The epidemiology of Fusarium wilt of banana. Front. Plant Sci. 2019, 10, 1395. [Google Scholar] [CrossRef] [Green Version]
- Edel-Hermann, V.; Lecomte, C. Current status of Fusarium oxysporum formae speciales and races. Phytopathology 2019, 109, 512–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giraldo-Betancourt, C.; Velandia-Sánchez, E.A.; Fischer, G.; Gómez-Caro, S.; Martínez, L.J. Hyperspectral response of cape gooseberry (Physalis peruviana L.) plants inoculated with Fusarium oxysporum f. sp. physali for vascular wilt detection. Rev. Colomb. Cienc. Hortic. 2020, 14, 301–313. [Google Scholar] [CrossRef]
- García-Bastidas, F.A.; Quintero-Vargas, J.C.; Ayala-Vasquez, M.; Schermer, T.; Seidl, M.F.; Santos-Paiva, M.; Noguera, A.M.; Aguilera-Galvez, C.; Wittenberg, A.; Hofstede, R.; et al. First report of Fusarium wilt tropical race 4 in cavendish bananas caused by Fusarium odoratissimum in Colombia. Plant Dis. 2020, 104, 994. [Google Scholar] [CrossRef]
- Basallote-Ureba, M.J.; Vela-Delgado, M.D.; Capote, N.; Melero-Vara, J.M.; López-Herrera, C.J.; Prados-Ligero, A.M.; Talavera-Rubia, M.F. Control of Fusarium wilt of carnation using organic amendments combined with soil solarization, and report of associated Fusarium species in southern Spain. Crop Prot. 2016, 89, 184–192. [Google Scholar] [CrossRef]
- Jangir, M.; Pathak, R.; Sharma, S.; Sharma, S. Biocontrol mechanisms of Bacillus sp., isolated from tomato rhizosphere, against Fusarium oxysporum f. sp. lycopersici. Biol. Control 2018, 123, 60–70. [Google Scholar] [CrossRef]
- Amini, J.; Dzhalilov, F.S. The effects of fungicides on Fusarium oxysporum f. sp. lycopersici associated with Fusarium wilt of tomato. J. Plant Prot. Res. 2010, 50, 172–178. [Google Scholar] [CrossRef]
- Ajilogba, C.F.; Babalola, O.O. Integrated management strategies for tomato Fusarium Wilt. Biocontrol Sci. 2013, 18, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Hernández Martínez, R.; López Benítez, A.; Borrego Escalante, F.; Espinoza Velázquez, J.; Sánchez Aspeytia, D.; Maldonado Mendoza, I.E.; López Ochoa, L.A. Races of Fusarium oxysporum f. sp. lycopersici in tomato farmlands in San Luis Potosí. Rev. Mex. Cienc. Agrícolas 2018, 5, 1169. [Google Scholar] [CrossRef] [Green Version]
- Petz, W.; Foissner, W. The effects of mancozeb and lindane on the soil microfauna of a spruce forest: A field study using a completely randomized block design. Biol. Fertil. Soils 1989, 7, 225–231. [Google Scholar] [CrossRef]
- Walia, A.; Mehta, P.; Guleria, S.; Chauhan, A.; Shirkot, C.K. Impact of fungicide mancozeb at different application rates on soil microbial populations, soil biological processes, and enzyme activities in soil. Sci. World J. 2014, 2014, 702909. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, K.N.; Buirchell, B.J.; Sweetingham, M.W. Length of vernalization period affects flowering time in three lupin species. Plant Breed. 2012, 131, 631–636. [Google Scholar] [CrossRef]
- Clapham, W.L.; Willcott, J.B. A Thermosensitivity index for Lupinus albus L. Crop Sci. 1999, 39, 578–580. [Google Scholar] [CrossRef]
- Podleśny, J.; Podleśna, A. The effect of high temperature during flowering on growth, development and yielding of blue lupine-barley mixture. J. Food Agric. Environ. 2012, 10, 500–504. [Google Scholar]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Przybylak, J.K.; Ciesiołka, D.; Wysocka, W.; García-López, P.M.; Ruiz-López, M.A.; Wysocki, W.; Gulewicz, K. Alkaloid profiles of Mexican wild lupin and an effect of alkaloid preparation from Lupinus exaltatus seeds on growth and yield of paprika (Capsicum annuum L.). Ind. Crop. Prod. 2005, 21, 1–7. [Google Scholar] [CrossRef]
- Cook, D.; Lee, S.T.; Gardner, D.R.; Pfister, J.A.; Welch, K.D.; Green, B.T.; Davis, T.Z.; Panter, K.E. The alkaloid profiles of Lupinus sulphureus. J. Agric. Food Chem. 2009, 57, 1646–1653. [Google Scholar] [CrossRef]
- McDougal, O.M.; Heenan, P.B.; Jaksons, P.; Sansom, C.E.; Smallfield, B.M.; Perry, N.B.; van Klink, J.W. Alkaloid variation in New Zealand kōwhai, Sophora species. Phytochemistry 2015, 118, 9–16. [Google Scholar] [CrossRef]
- Martínez-Herrera, J.; Robledo-Quintos, N.; Mora-Escobedo, R.; Dávila-Ortíz, G. Alkaloid composition of Lupinus campestris from Mexico. J. Food Biochem. 2001, 25, 117–125. [Google Scholar] [CrossRef]
- Kianbakht, S.; Dabaghian, F.H. Sophora alopecuroides L. var. alopecuroides alleviates morphine withdrawal syndrome in mice: Involvement of alkaloid fraction and matrine. Iran. J. Basic Med. 2016, 19, 1090–1095. [Google Scholar]
- Basilico, N.; Parapini, S.; Sparatore, A.; Romeo, S.; Misiano, P.; Vivas, L.; Yardley, V.; Croft, S.L.; Habluetzel, A.; Lucantoni, L.; et al. In vivo and in vitro activities and ADME-tox profile of a quinolizidine-modified 4-aminoquinoline: A potent anti-P. falciparum and Anti-P. vivax blood-stage antimalarial. Molecules 2017, 22, 2102. [Google Scholar] [CrossRef] [Green Version]
- Wink, M.; Carey, D.B. Variability of quinolizidine alkaloid profiles of Lupinus argentous (Fabaceae) from North America. Biochem. Syst. Ecol. 1994, 22, 663–669. [Google Scholar] [CrossRef]
- Adler, L.S.; Kittelson, P.M. Variation in Lupinus arboreus alkaloid profiles and relationships with multiple herbivores. Biochem. Syst. Ecol. 2004, 32, 371–390. [Google Scholar] [CrossRef] [Green Version]
- Kroc, M.; Rybiński, W.; Wilczura, P.; Kamel, K.; Kaczmarek, Z.; Barzyk, P.; Święcicki, W. Quantitative and qualitative analysis of alkaloids composition in the seeds of a white lupin (Lupinus albus L.) collection. Genet. Resour. Crop Evol. 2017, 64, 1853–1860. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Medina, L.; Yamaguchi, L.F.; Coy-Barrera, E. LC-ESI-HRMS-based Chemical characterization of Lupinus bogotensis roots. Rev. Fac. Cienc. Basic. 2016, 12, 200–211. [Google Scholar] [CrossRef]
- Wink, M.; Witte, L. Quinolizidine alkaloids in Genista acanthoclada and its holoparasite, Cuscuta palaestina. J. Chem. Ecol. 1993, 19, 441–448. [Google Scholar] [CrossRef]
- Kacem, N.; Goossens, J.-F.; Duhal, N.; Roumy, V.; Hennebelle, T.; Christen, P.; Hostettmann, K.; Rhouati, S. Determination of alkaloids in endemic Genista quadriflora Munby (Fabaceae). Biochem. Syst. Ecol. 2014, 56, 83–87. [Google Scholar] [CrossRef]
- Choma, I.; Jesionek, W. Effects-directed biological detection. In Instrumental Thin-Layer Chromatography; Elsevier: Amsterdam, The Netherlands, 2015; pp. 279–312. ISBN 9780124172845. [Google Scholar]
- Dewanjee, S.; Gangopadhyay, M.; Bhattacharya, N.; Khanra, R.; Dua, T.K. Bioautography and its scope in the field of natural product chemistry. J. Pharm. Anal. 2015, 5, 75–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamora-Natera, J.F.; Bernal-alcocer, A.; Ruiz-lópez, M.; Soto-hernández, M.; Vibrans-lindemann, H. Alkaloid profile of seeds from Lupinus exaltatus Zucc. (Fabaceae) and the antifungal evaluation of alkaloid extract and lupanin against phytopathogens. Rev. Mex. Fitopatol. 2005, 23, 124–129. [Google Scholar]
- Kwaśniewska, P.W.; Cofta, G.; Mazela, B.; Gobakken, L.R.; Przybył, A.K. Fungistatic activity of quinolizidine and bisquinolizidine alkaloids against A. niger. Int. Res. Gr. Wood Prot. 2016, 47, 1–9. [Google Scholar]
- Yang, X.; Zhao, B. Antifungal activities of matrine and oxymatrine and their synergetic effects with chlorthalonil. J. For. Res. 2006, 17, 323–325. [Google Scholar] [CrossRef]
- Zamora-Natera, F.; García-López, P.; Ruiz-López, M.; Salcedo-Pérez, E. Composition of alkaloids in seeds of Lupinus mexicanus (Fabaceae) and antifungal and allelopathic evaluation of the alkaloid extract. Agrociencia 2008, 42, 185–192. [Google Scholar]
- Pérez-Laínez, D.; García-Mateos, R.; San Miguel-Chávez, R.; Soto-Hernández, M.; Rodríguez-Pérez, E.; Kite, G. Bactericidal and fungicidal activities of Calia secundiflora (Ort.) Yakovlev. Z. Naturforsch. C 2008, 63, 653–657. [Google Scholar] [CrossRef]
- Shao, J.; Wang, T.; Yan, Y.; Shi, G.; Cheng, H.; Wu, D.; Wang, C. Matrine reduces yeast-to-hypha transition and resistance of a fluconazole-resistant strain of Candida albicans. J. Appl. Microbiol. 2014, 117, 618–626. [Google Scholar] [CrossRef]
- Goto, T.; Hirazawa, N.; Takaishi, Y.; Kashiwada, Y. Antiparasitic effect of matrine and oxymatrine (quinolizidine alkaloids) on the ciliate Cryptocaryon irritans in the red sea bream Pagrus major. Aquaculture 2015, 437, 339–343. [Google Scholar] [CrossRef]
- He, H.; Qin, X.; Dong, F.; Ye, J.; Xu, C.; Zhang, H.; Liu, Z.; Lv, X.; Wu, Y.; Jiang, X.; et al. Synthesis, characterization of two matrine derivatives and their cytotoxic effect on Sf9 cell of Spodoptera frugiperda. Sci. Rep. 2020, 10, 17999. [Google Scholar] [CrossRef]
- ur Rashid, H.; Xu, Y.; Muhammad, Y.; Wang, L.; Jiang, J. Research advances on anticancer activities of matrine and its derivatives: An updated overview. Eur. J. Med. Chem. 2019, 161, 205–238. [Google Scholar] [CrossRef]
- Yang, Y.; Xiu, J.; Zhang, X.; Zhang, L.; Yan, K.; Qin, C.; Liu, J. Antiviral effect of matrine against human enterovirus 71. Molecules 2012, 17, 10370–10376. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Huang, C.F.; Liu, X.S.; Jiang, J. In vitro anti-tumour activities of quinolizidine alkaloids derived from Sophora flavescens Ait. Basic Clin. Pharmacol. Toxicol. 2011, 108, 304–309. [Google Scholar] [CrossRef]
- Liu, W.; Shi, J.; Zhu, L.; Dong, L.; Luo, F.; Zhao, M.; Wang, Y.; Hu, M.; Lu, L.; Liu, Z. Reductive metabolism of oxymatrine is catalyzed by microsomal CYP3A4. Drug Des. Devel. Ther. 2015, 9, 5771–5783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrenk, D.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.-C.; Nebbia, C.S.; Nielsen, E.; et al. Scientific opinion on the risks for animal and human health related to the presence of quinolizidine alkaloids in feed and food, in particular in lupins and lupin-derived products. Eur. Food Saf. Auth. J. 2019, 17, e05860. [Google Scholar]
- Green, B.T.; Lee, S.T.; Welch, K.D.; Cook, D. Anagyrine desensitization of peripheral nicotinic acetylcholine receptors. A potential biomarker of quinolizidine alkaloid teratogenesis in cattle. Res. Vet. Sci. 2017, 115, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Babushok, V.I. Chromatographic retention indices in identification of chemical compounds. TrAC—Trends Anal. Chem. 2015, 69, 98–104. [Google Scholar] [CrossRef]
- Marques, J.V.; Kitamura, R.O.S.; Lago, J.H.G.; Young, M.C.M.; Guimarães, E.F.; Kato, M.J. Antifungal amides from Piper scutifolium and Piper hoffmanseggianum. J. Nat. Prod. 2007, 70, 2036–2039. [Google Scholar] [CrossRef] [PubMed]
- Marentes-Culma, R.; Orduz-Díaz, L.L.; Coy-Barrera, E. Targeted metabolite profiling-based identification of antifungal 5-n-alkylresorcinols occurring in different cereals against Fusarium oxysporum. Molecules 2019, 24, 770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, M.D. Key antifungal, antibacterial and anti-insect assays—A critical review. Biochem. Syst. Ecol. 1994, 22, 837–856. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Version 24. Available online: http://www.infostat.com.ar/ (accessed on 12 November 2021).







| # a | Rt b (min) | Name | RI c | RI d | Reference d |
|---|---|---|---|---|---|
| 1 | 20.5 | sparteine | 1785 | 1805 | [39] |
| 2 | 21.6 | 11,12-dehydrosparteine | 1841 | 1841 | [40] |
| 3 | 24.4 | N-methylcytisine | 1918 | 1924 | [41] |
| 4 | 25.8 | angustifoline | 2079 | 2073 | [39] |
| 5 | 26.3 | 5,6-dehydrolupanine | 2104 | 2092 | [40] |
| 6 | 27.1 | α-isolupanine | 2107 | 2123 | [39] |
| 7 | 27.7 | lupanine | 2170 | 2146 | [42] |
| 8 | 29.0 | nuttalline | 2338 | 2348 | [18] |
| 9 | 30.9 | matrine | 2366 | 2365 | [43] |
| 10 | 31.1 | anagyrine | 2390 | 2377 | [40] |
| 11 | 31.4 | 13α-hydroxylupanine | 2405 | 2400 | [39] |
| 12 | 35.7 | multiflorine | 2440 | 2469 | [18] |
| 13 | 36.0 | 17-oxolupanine | 2482 | 2473 | [18] |
| Plants | QA Content (mg LE/g FL) a | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| L.po. | 1.43 ± 0.12 C | n.d. | n.d. | 5.39 ± 0.18 A | 0.391 ± 0.008 B | 0.169 ± 0.009 E | 17.1 ± 0.9 C |
| L.pe. | n.d. | 0.031 ± 0.001 D | n.d. | 3.12 ± 0.12 B | n.d. | 1.607 ± 0.032 B | 20.0 ± 0.2 B |
| L.b. | 22.4 ± 0.7 B | 0.033 ± 0.002 D | n.d. | n.d. | n.d. | 0.083 ± 0.002 F | 19.3 ± 0.8 B |
| L.mu. | n.d. | n.d. | n.d. | 0.87 ± 0.09 C | 1.42 ± 0.16 A | 0.452 ± 0.009 C | 25.7 ± 0.6 A |
| L.mi. | 32.8 ± 0.6 A | 0.102 ± 0.004 C | n.d. | 0.102 ± 0.008 D | n.d. | 0.151 ± 0.006 E | 20.1 ± 0.31 B |
| L.arg. | n.d. | 1.38 ± 0.19 B | 4.98 ± 0.19 B | 0.051 ± 0.003 E | n.d. | 0.201 ± 0.016 D | 5.62 ± 0.14 F |
| L.arb | n.d. | 2.07 ± 0.16 A | 5.69 ± 0.21 A | 0.046 ± 0.002 E | n.d. | 0.046 ± 0.003 G | 6.56 ± 0.20 G |
| L.al. | n.d. | n.d. | n.d. | 3.35 ± 0.14 B | n.d. | 3.12 ± 0.06 A | 13.8 ± 0.9 D |
| Plants | QA Content (mg LE/g FL) a | ||||||
| 8 | 9 | 10 | 11 | 12 | 13 | ||
| L.po. | 1.08 ± 0.09 C | 3.54 ± 0.08 A | n.d. | 2.62 ± 0.09 A | 0.328 ± 0.013 C | 9.40 ± 0.75 A | |
| L.pe. | 0.735 ± 0.059 D | 0.043 ± 0.003 C | 0.048 ± 0.001 C | 0.068 ± 0.002 D | 0.154 ± 0.005 E | 4.09 ± 0.11 C | |
| L.b. | 5.41 ± 0.16 B | n.d. | n.d. | n.d. | 0.209 ± 0.017 D | 0.051 ± 0.005 F | |
| L.mu. | n.d. | 0.729 ± 0.008 B | n.d. | 0.727 ± 0.011 B | 0.812 ± 0.012 B | 4.98 ± 0.23 B | |
| L.mi. | 0.176 ± 0.011 E | n.d. | n.d. | n.d. | 0.045 ± 0.002 F | 0.063 ± 0.006 F | |
| L.arg. | n.d. | n.d. | 6.08 ± 0.07 A | n.d. | 4.88 ± 0.34 A | n.d. | |
| L.arb | n.d. | n.d. | 4.61 ± 0.13 B | n.d. | n.d. | 0.104 ± 0.008 E | |
| L.al. | 6.15 ± 0.08 A | n.d. | n.d. | 0.377 ± 0.011 C | 0.910 ± 0.017 B | 4.97 ± 0.21 B | |
| PE a | QA Content (mg LE/g FL) b | ||||
|---|---|---|---|---|---|
| 1 | 4 | 5 | 6 | 7 | |
| PE1 | 0.011 ± 0.001 F | 0.123 ± 0.011 G | 1.73 ± 0.09 G | 1.07 ± 0.08 E | 11.3 ± 0.4 E |
| PE2 | 0.009 ± 0.001 F | 0.115 ± 0.003 G | 2.77 ± 0.22 EF | 1.12 ± 0.09 E | 14.8 ± 0.4 C |
| PE3 | 0.014 ± 0.001 F | 0.130 ± 0.009 FG | 1.42 ± 0.11 G | 1.70 ± 0.12 C | 12.7 ± 0.3 D |
| PE4 | 0.016 ± 0.001 E | 0.269 ± 0.011 E | 3.57 ± 0.14 D | 0.90 ± 0.06 F | 13.7 ± 0.7 CD |
| PE5 | 0.118 ± 0.006 D | 0.341 ± 0.017 D | 5.32 ± 0.08 B | 1.39 ± 0.04 D | 18.6 ± 0.6 A |
| PE6 | 0.184 ± 0.015 C | 0.094 ± 0.003 G | 8.72 ± 0.09 A | 3.36 ± 0.22 B | 14.8 ± 0.8 C |
| PE7 | 0.375 ± 0.004 A | 0.137 ± 0.001 F | 8.93 ± 0.17 A | 5.15 ± 0.10 A | 16.9 ± 0.7 B |
| PE8 | 0.282 ± 0.006 B | 0.139 ± 0.003 F | 8.52 ± 0.15 A | 3.57 ± 0.21 B | 15.5 ± 0.5 C |
| PE9 | 0.131 ± 0.007 D | 0.003 ± 0.001 H | 5.04 ± 0.21 B | 0.009 ± 0.001 G | 13.4 ± 0.8 CD |
| PE10 | tr. | 1.602 ± 0.128 B | 4.49 ± 0.14 C | 1.12 ± 0.05 E | 11.7 ± 0.5 E |
| PE11 | tr. | 1.360 ± 0.054 C | 2.91 ± 0.12 E | 1.67 ± 0.08 C | 13.2 ± 0.6 D |
| PE12 | n.d. | 4.026 ± 0.081 A | 2.43 ± 0.15 F | tr. | 13.4 ± 0.7 CD |
| PE a | QA Content (mg LE/g FL) b | ||||
| 8 | 9 | 11 | 12 | 13 | |
| PE1 | tr. | tr. | n.d. | 0.055 ± 0.002 B | 1.74 ± 0.10 F |
| PE2 | tr. | 0.014 ± 0.001 F | n.d. | 0.076 ± 0.003 A | 1.86 ± 0.15 EF |
| PE3 | tr. | 0.162 ± 0.008 B | n.d. | tr. | 1.42 ± 0.08 G |
| PE4 | tr. | 0.132 ± 0.009 C | n.d. | tr. | 1.57 ± 0.09 FG |
| PE5 | 0.068 ± 0.003 B | 0.051 ± 0.006 E | n.d. | tr. | 1.65 ± 0.13 F |
| PE6 | 0.004 ± 0.001 C | 0.074 ± 0.005 D | n.d. | n.d. | 4.84 ± 0.21 B |
| PE7 | 0.003 ± 0.001 C | 0.201 ± 0.011 A | tr. | n.d. | 14.7 ± 0.5 A |
| PE8 | 0.149 ± 0.012 A | 0.088 ± 0.001 D | tr. | n.d. | 14.3 ± 0.4 A |
| PE9 | tr. | n.d. | tr. | n.d. | 2.47 ± 0.19 D |
| PE10 | n.d. | n.d. | 1.69 ± 0.07 A | n.d. | 2.10 ± 0.14 E |
| PE11 | n.d. | n.d. | 1.52 ± 0.06 B | n.d. | 4.01 ± 0.18 C |
| PE12 | n.d. | n.d. | 0.039 ± 0.005 C | n.d. | 4.53 ± 0.19 BC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cely-Veloza, W.; Quiroga, D.; Coy-Barrera, E. Quinolizidine-Based Variations and Antifungal Activity of Eight Lupinus Species Grown under Greenhouse Conditions. Molecules 2022, 27, 305. https://doi.org/10.3390/molecules27010305
Cely-Veloza W, Quiroga D, Coy-Barrera E. Quinolizidine-Based Variations and Antifungal Activity of Eight Lupinus Species Grown under Greenhouse Conditions. Molecules. 2022; 27(1):305. https://doi.org/10.3390/molecules27010305
Chicago/Turabian StyleCely-Veloza, Willy, Diego Quiroga, and Ericsson Coy-Barrera. 2022. "Quinolizidine-Based Variations and Antifungal Activity of Eight Lupinus Species Grown under Greenhouse Conditions" Molecules 27, no. 1: 305. https://doi.org/10.3390/molecules27010305
APA StyleCely-Veloza, W., Quiroga, D., & Coy-Barrera, E. (2022). Quinolizidine-Based Variations and Antifungal Activity of Eight Lupinus Species Grown under Greenhouse Conditions. Molecules, 27(1), 305. https://doi.org/10.3390/molecules27010305