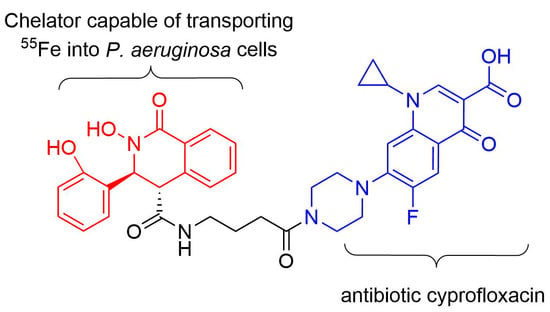
Conjugates of Iron-Transporting N-Hydroxylactams with Ciprofloxacin
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Bacterial Strains and Growth Assays in Iron-Restricted Conditions
3.2. Iron Uptake
3.3. MIC Determination
3.4. Compounds Synthesis
3.4.1. (3RS,4RS)-2-Hydroxy-3-(2-hydroxyphenyl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (4)
3.4.2. (3RS,4RS)-2-(Benzyloxy)-3-(2-methoxyphenyl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (5b)
3.4.3. (3RS,4RS)-2-(Benzyloxy)-3-(2-fluorophenyl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (5c)
3.4.4. 4-((3RS,4RS)-2-(Benzyloxy)-3-(4-methoxyphenyl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxamido)butanoic acid (6a)
3.4.5. 4-((3RS,4RS)-2-(Benzyloxy)-3-(2-methoxyphenyl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxamido)butanoic acid (6b)
3.4.6. 4-((3RS,4RS)-2-(Benzyloxy)-3-(2-fluorophenyl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxamido)butanoic acid (6c)
3.4.7. Methyl 7-(4-(4-((3RS,4RS)-2-(benzyloxy)-3-(4-methoxyphenyl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxamido)butanoyl)piperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (7a)
3.4.8. Benzyl 7-(4-(4-((3RS,4RS)-2-(benzyloxy)-3-(2-methoxyphenyl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxamido)butanoyl)piperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (7b)
3.4.9. 7-(4-(4-((3SR,4SR)-2-(Benzyloxy)-3-(2-fluorophenyl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxamido)butanoyl)piperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (7c)
3.4.10. 1-Cyclopropyl-6-fluoro-7-(4-(4-((3RS,4RS)-2-hydroxy-3-(4-methoxyphenyl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxamido)butanoyl)piperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (8a)
3.4.11. 1-Cyclopropyl-6-fluoro-7-(4-(4-((3RS,4RS)-2-hydroxy-3-(2-hydroxyphenyl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxamido)butanoyl)piperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (8b)
3.4.12. 1-Cyclopropyl-6-fluoro-7-(4-(4-((3SR,4SR)-2-hydroxy-3-(2-methoxyphenyl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxamido)butanoyl)piperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (8c)
3.4.13. 1-Cyclopropyl-6-fluoro-7-(4-(4-((3S,4S)-3-(2-fluorophenyl)-2-hydroxy-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxamido)butanoyl)piperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (8d)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Schalk, I.J.; Hannauer, M.; Braud, A. New roles for bacterial siderophores in metal transport and tolerance. Environ. Microbiol. 2011, 13, 2844–2854. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.; Saha, N.; Donofrio, R.S.; Bestervelt, L.L. Microbial siderophores: A mini review. J. Basic Microbiol. 2013, 53, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Sontag, B.; Gerlitz, M.; Paululat, T.; Rasser, H.F.; Grun-Wollny, I.; Hansske, F.G. Oxachelin, a Novel Iron Chelator and Antifungal Agent from Streptomyces sp. GW9/1258. J. Antibiot. 2006, 59, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Kodani, S.; Bicz, J.; Song, L.; Deeth, R.J.; Ohnishi-Kameyama, M.; Yoshida, M.; Ochi, K.; Challis, G.L. Structure and biosynthesis of scabichelin, a novel tris-hydroxamate siderophore produced by the plant pathogen Streptomyces scabies 87.22. Org. Biomol. Chem. 2013, 11, 4686–4694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grӓfe, U.; Ritzau, M.; Ihn, W.; Möllmann, U.; Fleck, W.F.; Groth, J.; Reissbrodt, R. A new microbial isoquinoline iron chelator from Streptomyces spec. 2002-104. J. Basic Microbiol. 1994, 34, 351–355. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Xiang, B.; Yang, S.-M.; Rhodes, K.; Scannevin, R.; Jackson, P.; Chakravarty, D.; Fan, X.; Wilson, L.J.; Karnachi, P. Heterocyclic Derived Metalloprotease Inhibitors. PCT Int. Appl. WO 2008045668. Chem. Abstr. 2008, 148, 471882. [Google Scholar]
- Zhang, Y.-M.; Fan, X.; Yang, S.-M.; Scannevin, R.H.; Burke, S.L.; Rhodes, K.J.; Jackson, P.F. Syntheses and in vitro evaluation of arylsulfone-based MMP inhibitors with heterocycle-derived zinc-binding groups (ZBGs). Bioorg. Med. Chem. Lett. 2008, 18, 405–408. [Google Scholar] [CrossRef]
- Pryde, D.C.; Webster, R.; Butler, S.L.; Murray, E.J.; Whitby, K.; Pickford, C.; Westby, M.; Palmer, M.J.; Bull, D.J.; Vuong, H.; et al. Discovery of an HIV integrase inhibitor with an excellent resistance profile. MedChemComm 2013, 4, 709–719. [Google Scholar] [CrossRef]
- Mutule, I.; Borovika, D.; Rozenberga, E.; Romanchikova, N.; Zalubovskis, R.; Shestakova, I.; Trapencieris, P. 5-Membered cyclic hydroxamic acids as HDAC inhibitors. J. Enzyme Inhib. Med. Chem. 2015, 30, 216–223. [Google Scholar] [CrossRef]
- Tang, D.G.; Li, L.; Zhu, Z.; Joshi, B.; Johnson, C.R.; Marnett, L.J.; Honn, K.V.; Crissman, J.D.; Krajewski, S.; Reed, J.C.; et al. BMD188, A novel hydroxamic acid compound, demonstrates potent anti-prostate cancer effectsin vitro andin vivo by inducing apoptosis: Requirements for mitochondria, reactive oxygen species, and proteases. Pathol. Oncol. Res. 1998, 4, 179–190. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Nolan, E.M. Beyond iron: Non-classical biological functions of bacterial siderophores. Dalton Trans. 2015, 44, 6320–6339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dassonville-Klimpt, A.; Sonnet, P. Advances in ‘Trojan horse’ strategies in antibiotic delivery systems. Future Med. Chem. 2022, 12, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Schalk, I.J. A Trojan-Horse Strategy Including a Bacterial Suicide Action for the Efficient Use of a Specific Gram-Positive Antibiotic on Gram-Negative Bacteria. J. Med. Chem. 2018, 61, 3842–3844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, K.; Hu, H.-Y.; Fetz, V.; Prochnow, H.; Rais, B.; Müller, P.P.; Brönstrup, M. Multivalent Siderophore-DOTAM Conjugates as Theranostics for Imaging and Treatment of Bacterial Infections. Angew. Chem. Int. Ed. 2017, 56, 8272–8276. [Google Scholar] [CrossRef]
- Katsube, T.; Echols, R.; Ferreira, J.C.A.; Krenz, H.K.; Berg, J.K.; Galloway, C. Cefiderocol, a Siderophore Cephalosporin for Gram-Negative Bacterial Infections: Pharmacokinetics and Safety in Subjects With Renal Impairment. J. Clin. Pharmacol. 2017, 57, 584–591. [Google Scholar] [CrossRef]
- Bakulina, O.; Bannykh, A.; Dar’in, D.; Krasavin, M. Cyclic Hydroxamic Acid Analogues of Bacterial Siderophores as Iron-Complexing Agents prepared through the Castagnoli–Cushman Reaction of Unprotected Oximes. Chem. Eur. J. 2017, 23, 17667–17673. [Google Scholar] [CrossRef]
- Bannykh, A.V.; Bakulina, O.Y.; Dar’in, D.V.; Krasavin, M. Hydroxylamine as an ammonia equivalent: Access to NH-tetrahydroisoquinolonic derivatives from aldoximes by the Castagnoli–Cushman reaction followed by reduction. Mendeleev Commun. 2019, 29, 337–338. [Google Scholar] [CrossRef]
- Chupakhin, E.; Bakulina, O.; Dar’in, D.; Krasavin, M. Facile Access to Fe(III)-Complexing Cyclic Hydroxamic Acids in a Three-Component Format. Molecules 2019, 24, 864. [Google Scholar] [CrossRef] [Green Version]
- Negash, K.H.; Norris, J.K.S.; Hodgkinson, J.T. Siderophore–Antibiotic Conjugate Design: New Drugs for Bad Bugs? Molecules 2019, 24, 3314. [Google Scholar] [CrossRef] [Green Version]
- Sanderson, T.J.; Black, C.M.; Southwell, J.W.; Wilde, E.J.; Pandey, A.; Herman, R.; Thomas, G.H.; Boros, E.; Duhme-Klair, A.-K.; Routledge, A. A Salmochelin S4-Inspired Ciprofloxacin Trojan Horse Conjugate. ACS Infect. Dis. 2020, 6, 2532. [Google Scholar] [CrossRef]
- Cunrath, O.; Gasser, V.; Hoegy, F.; Reimmann, C.; Guillon, L.; Schalk, I. A cell biological view of the siderophore pyochelin iron uptake pathway in Pseudomonas aeruginosa. J. Environ. Microbiol. 2015, 17, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Menhart, N.; Thariath, A.; Viswanatha, T. Characterization of the pyoverdines of Azotobacter vinelandii ATCC 12 837 with regard to heterogeneity. Biol. Metals 1991, 4, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Brandel, J.; Humbert, N.; Elhabiri, M.; Schalk, I.J.; Mislin, G.L.A.; Albrecht-Gary, A.-M. Pyochelin, a siderophore of Pseudomonas aeruginosa: Physicochemical characterization of the iron(iii), copper(ii) and zinc(ii) complexes. Dalton Trans. 2012, 41, 2820–2834. [Google Scholar] [CrossRef] [PubMed]
- Gasser, V.; Baco, E.; Cunrath, O.; Saint August, P.; Perraud, Q.; Zill, N.; Schleberger, C.; Schmidt, A.; Paulen, A.; Bumann, D.; et al. Catechol siderophores repress the pyochelin pathway and activate the enterobactin pathway in Pseudomonas aeruginosa: An opportunity for siderophore–antibiotic conjugates development. J. Environ. Microbiol. 2016, 18, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.; Stintzi, A.; Gilmour, C.; Meyer, J.-M.; Poole, K. Citrate-mediated iron uptake in Pseudomonas aeruginosa: Involvement of the citrate-inducible FecA receptor and the FeoB ferrous iron transporter. Microbiology 2009, 155, 305–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, C.K.Y.; Krewulak, K.D.; Vogel, H.J. Bacterial ferrous iron transport: The Feo system. FEMS Microbiol. Rev. 2016, 40, 273–298. [Google Scholar] [CrossRef] [PubMed]
- Woronowicz, K.; Olubanjo, O.B.; Sha, D.; Kay, J.M.; Niederman, R.A. Effects of the protonophore carbonyl-cyanide m-chlorophenylhydrazone on intracytoplasmic membrane assembly in Rhodobacter sphaeroides. Biochim. Biophys. Acta 2015, 1847, 1119–1128. [Google Scholar] [CrossRef] [Green Version]
- Noinaj, N.; Guillier, M.; Barnard, T.J.; Buchanan, S.K. TonB-Dependent Transporters: Regulation, Structure, and Function. Annu. Rev. Microbiol. 2010, 64, 43–60. [Google Scholar] [CrossRef] [Green Version]
- Clément, E.; Mesini, P.J.; Pattus, F.; Abdallah, M.A.; Schalk, I. The binding mechanism of pyoverdin with the outer membrane receptor FpvA in Pseudomonas aeruginosa is dependent on its iron-loaded status. J. Biochemistry 2004, 43, 7954–7965. [Google Scholar] [CrossRef]
- Perraud, Q.; Cantero, P.; Munier, M.; Hoegy, F.; Zill, N.; Gasser, V.; Mislin, G.L.A.; Ehret-Sabatier, L.; Schalk, I. Phenotypic Adaptation of Pseudomonas aeruginosa in the Presence of Siderophore-Antibiotic Conjugates during Epithelial Cell Infection. J. Microorganisms 2020, 8, 1820. [Google Scholar] [CrossRef]
- Schirmer, T. General and Specific Porins from Bacterial Outer Membranes. J. Struct. Biol. 1998, 121, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Wencewicz, T.A.; Mollmann, U.; Long, T.E.; Miller, M.J. Syntheses and biological studies of the naturally occurring salmycin “Trojan Horse” antibiotics and synthetic desferridanoxamine-antibiotic conjugates. Biometals 2009, 22, 633–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, J.H.; Hinton, A. Protein-Free Medium for Primary Isolation of the Gonococcus and Meningococcus. J. Exp. Biol. Med. 1941, 48, 330–333. [Google Scholar] [CrossRef]





| Compound | MH Medium | CAA Medium | ||
|---|---|---|---|---|
| PAO1 | ∆pvdF∆pchA | PAO1 | ∆pvdF∆pchA | |
| 8a | >64 | 64 | >64 | 1 |
| 8b | >64 | 64 | >64 | 1 |
| 8c | >64 | 64 | >64 | 1 |
| 8d | >64 | 64 | >64 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakulina, O.; Bannykh, A.; Levashova, E.; Krasavin, M. Conjugates of Iron-Transporting N-Hydroxylactams with Ciprofloxacin. Molecules 2022, 27, 3910. https://doi.org/10.3390/molecules27123910
Bakulina O, Bannykh A, Levashova E, Krasavin M. Conjugates of Iron-Transporting N-Hydroxylactams with Ciprofloxacin. Molecules. 2022; 27(12):3910. https://doi.org/10.3390/molecules27123910
Chicago/Turabian StyleBakulina, Olga, Anton Bannykh, Ekaterina Levashova, and Mikhail Krasavin. 2022. "Conjugates of Iron-Transporting N-Hydroxylactams with Ciprofloxacin" Molecules 27, no. 12: 3910. https://doi.org/10.3390/molecules27123910
APA StyleBakulina, O., Bannykh, A., Levashova, E., & Krasavin, M. (2022). Conjugates of Iron-Transporting N-Hydroxylactams with Ciprofloxacin. Molecules, 27(12), 3910. https://doi.org/10.3390/molecules27123910