Antibacterial Activity of a Promising Antibacterial Agent: 22-(4-(2-(4-Nitrophenyl-piperazin-1-yl)-acetyl)-piperazin-1-yl)-22-deoxypleuromutilin
Abstract
:1. Introduction
2. Results
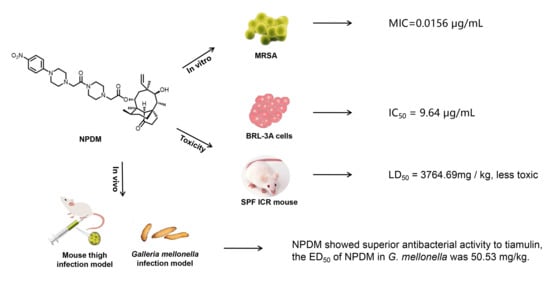
2.1. In Vitro Efficacy of NPDM
2.2. Toxicity Determination
2.3. In Vivo Effect of NPDM
2.4. Pharmacokinetic Study
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture and Preparation
4.3. Bacterial Strains and Culture Medium
4.4. Animal
4.5. Antimicrobial Activity Evaluation
4.5.1. The MICs Assay
4.5.2. The MBCs Assay
4.5.3. Time–Kill Assay
4.5.4. The PAE Assay
4.6. Toxicity Determination
4.6.1. Cytotoxicity Assay
4.6.2. Acute Oral Toxicity Evaluation
4.7. Animal In Vivo Model
4.7.1. Mouse Thigh Infection Model
4.7.2. Mouse Systemic Infection Model
4.7.3. Galleria Mellonella Infection Model
4.8. Pharmacokinetic Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Diaz, R.; Ramalheira, E.; Afreixo, V.; Gago, B. Methicillin-resistant Staphylococcus aureus carrying the new mecC gene—A meta-analysis. Diagn. Microbiol. Infect. Dis. 2016, 84, 135–140. [Google Scholar] [CrossRef]
- Moellering, R.C., Jr. MRSA: The first half century. J. Antimicrob. Chemother. 2012, 67, 4–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frazee, B.W.; Lynn, J.; Charlebois, E.D.; Lambert, L.; Lowery, D.; Perdreau-Remington, F. High Prevalence of Methicillin-Resistant Staphylococcus aureus in Emergency Department Skin and Soft Tissue Infections. Ann. Emerg. Med. 2005, 45, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Nyasulu, P.; Chipolombwe, J.; Török, M.E.; Mbelle, N. Methicillin-resistant Staphylococcus aureus multiple sites surveillance: A systemic review of the literature. Infect. Drug Resist. 2016, 9, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Kaku, N.; Yanagihara, K.; Morinaga, Y.; Yamada, K.; Harada, Y.; Migiyama, Y.; Nagaoka, K.; Matsuda, J.-I.; Uno, N.; Hasegawa, H.; et al. Influence of antimicrobial regimen on decreased in-hospital mortality of patients with MRSA bacteremia. J. Infect. Chemother. 2014, 20, 350–355. [Google Scholar] [CrossRef] [Green Version]
- Kavanagh, F.; Hervey, A.; Robbins, W.J. Antibiotic Substances from Basidiomycetes: VIII. Pleurotus Multilus (Fr.) Sacc. and Pleurotus Passeckerianus Pilat. Proc. Nat. Acad. Sci. USA 1951, 37, 570–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.-Z.; Liu, Y.-H.; Chen, J.-X. Pleuromutilin and its derivatives-the lead compounds for novel antibiotics. Mini-Rev. Med. Chem. 2012, 12, 53–61. [Google Scholar] [CrossRef]
- Schlünzen, F.; Pyetan, E.; Fucini, P.; Yonath, A.; Harms, J.M. Inhibition of peptide bond formation by pleuromutilins: The structure of the 50S ribosomal subunit from Deinococcus radiodurans in complex with tiamulin. Mol. Microbiol. 2004, 54, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Dreier, I.; Hansen, L.H.; Nielsen, P.; Vester, B. A click chemistry approach to pleuromutilin derivatives. Part 3: Extended footprinting analysis and excellent MRSA inhibition for a derivative with an adenine phenyl side chain. Bioorg. Med. Chem. Lett. 2014, 24, 1043–1046. [Google Scholar] [CrossRef]
- Zhang, Z.-S.; Huang, Y.-Z.; Luo, J.; Wang, B.-F.; Jin, Z.; Liu, Y.-H.; Tang, Y.-Z. Synthesis and Antibacterial Activity against MRSA of Pleuromutilin Derivatives Possessing a Mercaptoethylamine Linker. Med. Chem. 2018, 14, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Fu, L.; Gao, S.; Chu, W.; Wang, H.; Huang, Y.; Chen, X.; Yang, Y. Design, Synthesis, and Structure–Activity Relationship Studies of Novel Thioether Pleuromutilin Derivatives as Potent Antibacterial Agents. J. Med. Chem. 2014, 57, 4772–4795. [Google Scholar] [CrossRef]
- Jones, R.N.; Fritsche, T.R.; Sader, H.S.; Ross, J.E. Activity of Retapamulin (SB-275833), a Novel Pleuromutilin, against Selected Resistant Gram-Positive Cocci. Antimicrob. Agents Chemother. 2006, 50, 2583–2586. [Google Scholar] [CrossRef] [Green Version]
- Malani, P.N. Lefamulin—A New Antibiotic for Community-Acquired Pneumonia. JAMA 2019, 322, 1671–1672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, X.; Fang, X.; Zhang, Z.; Jin, Z.; Xi, G.; Liu, Y.; Tang, Y. Antibacterial Activity and Pharmacokinetic Profile of a Promising Antibacterial Agent: 22-(2-Amino-phenylsulfanyl)-22-Deoxypleuromutilin. Molecules 2020, 25, 878. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.-L.; Zeng, J.; Fang, X.; Luo, J.; Jin, Z.; Liu, Y.-H.; Tang, Y.-Z. Design, synthesis and antibacterial evaluation of novel pleuromutilin derivatives possessing piperazine linker. Eur. J. Med. Chem. 2017, 127, 286–295. [Google Scholar] [CrossRef]
- Farrell, D.J.; Mendes, R.E.; Rhomberg, P.R.; Jones, R.N. Revised reference broth microdilution method for testing telavancin: Effect on MIC results and correlation with other testing methodologies. Antimicrob. Agents Chemother. 2014, 58, 5547–5551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, M.; De La Peña, A.; Derendorf, H. Issues in Pharmacokinetics and Pharmacodynamics of Anti-Infective Agents: Kill Curves versus MIC. Antimicrob. Agents Chemother. 2004, 48, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Plachouras, D.; Giamarellos-Bourboulis, E.J.; Kentepozidis, N.; Baziaka, F.; Karagianni, V.; Giamarellou, H. In vitro postantibiotic effect of colistin on multidrug-resistant Acinetobacter baumannii. Diagn. Microbiol. Infect. Dis. 2007, 57, 419–422. [Google Scholar] [CrossRef]
- Dadarkar, S.; Deore, M.; Gatne, M. Comparative evaluation of acute toxicity of ivermectin by two methods after single subcutaneous administration in rats. Regul. Toxicol. Pharmacol. 2007, 47, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yi, Y.; Chen, J.; Xin, R.; Yang, Z.; Guo, Z.; Liang, J.; Shang, R. In Vivo Efficacy and Toxicity Studies of a Novel Antibacterial Agent: 14-O-[(2-Amino-1,3,4-thiadiazol-5-yl)Thioacetyl] Mutilin. Molecules 2015, 20, 5299–5312. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Lepak, A.J.; Andes, D.R. Animal models in the pharmacokinetic/pharmacodynamic evaluation of antimicrobial agents. Bioorg. Med. Chem. 2016, 24, 6390–6400. [Google Scholar] [CrossRef]
- Staniszewska, M.; Gizińska, M.; Mikulak, E.; Adamus, K.; Koronkiewicz, M.; Łukowska-Chojnacka, E. New 1,5 and 2,5-disubstituted tetrazoles-dependent activity towards surface barrier of Candida albicans. Eur. J. Med. Chem. 2018, 145, 124–139. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.J.-Y.; Loh, J.M.S.; Proft, T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 2016, 7, 214–229. [Google Scholar] [CrossRef] [Green Version]
- Zadrazilova, I.; Pospisilova, S.; Masarikova, M.; Imramovsky, A.; Ferriz, J.M.; Vinsova, J.; Cizek, A.; Jampilek, J. Salicylanilide carbamates: Promising antibacterial agents with high in vitro activity against methicillin-resistant Staphylococcus aureus (MRSA). Eur. J. Pharm. Sci. 2015, 77, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Shang, R.; Yi, Y.; Zhang, C.; Fu, Y.; Liang, J.; Pu, W. Antibacterial activity and pharmacokinetic profile of a promising antibacterial agent: 14-O-[(4-Amino-6-hydroxy-pyrimidine-2-yl)thioacetyl] mutilin. Pharmacol. Res. 2018, 129, 424–431. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, L.; Gao, H.; Zhou, Y.-H.; Liu, Y.-H.; Tang, Y.-Z. Design, synthesis and biological evaluation of novel pleuromutilin derivatives possessing acetamine phenyl linker. Eur. J. Med. Chem. 2019, 181, 111594. [Google Scholar] [CrossRef] [PubMed]
- Fei, C.; She, R.; Li, G.; Zhang, L.; Fan, W.; Xia, S.; Xue, F. Safety and clinical efficacy of tenvermectin, a novel antiparasitic 16-membered macrocyclic lactone antibiotics. Eur. J. Pharm. Sci. 2018, 117, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Wang, X.; Wang, C.; Wang, M.; Fei, C.; Zhang, L.; Xue, F.; Wang, G.; Zhang, K. Acute and 30-day oral toxicity studies of a novel coccidiostat—ethanamizuril. Toxicol. Res. 2019, 8, 686–695. [Google Scholar] [CrossRef]
- Li, Y.; Lin, X.; Yao, X.; Huang, Y.; Liu, W.; Ma, T.; Fang, B. Synergistic Antimicrobial Activity of Colistin in Combination with Rifampin and Azithromycin against Escherichia coli Producing MCR-1. Antimicrob. Agents Chemother. 2018, 62, e01631-18. [Google Scholar] [CrossRef] [Green Version]
- Li, C.-R.; Li, Y.; Li, G.-Q.; Yang, X.-Y.; Zhang, W.-X.; Lou, R.-H.; Liu, J.-F.; Yuan, M.; Huang, P.; Cen, S.; et al. In vivo antibacterial activity of nemonoxacin, a novel non-fluorinated quinolone. J. Antimicrob. Chemother. 2010, 65, 2411–2415. [Google Scholar] [CrossRef] [Green Version]








| Compounds | MICs/MBCs (μg/mL) | |||
|---|---|---|---|---|
| ATCC 43300 | ATCC 29213 | AD3 | 144 | |
| NPDM | 0.125/0.125 | 0.125/0.25 | 0.125/0.5 | 0.125/0.5 |
| Tiamulin | 0.5/0.5 | 0.5/0.5 | 0.5/2 | 0.5/2 |
| Compounds | Concentrations | PAE (h) | |
|---|---|---|---|
| Exposure for 1 h | Exposure for 2 h | ||
| NPDM | 2 × MIC | 2.58 | 3.62 |
| 4 × MIC | 3.11 | 4.03 | |
| Tiamulin | 2 × MIC | 1.53 | 1.65 |
| 4 × MIC | 1.90 | 2.04 | |
| Group | n | Dose (mg/kg b.w.) | Logarithmic Dose | Mortality | Mortality Rate (%) |
|---|---|---|---|---|---|
| 1 | 10 | 987.65 | 3.70 | 1 | 10 |
| 2 | 10 | 1481.48 | 3.52 | 1 | 10 |
| 3 | 10 | 2222.22 | 3.35 | 2 | 20 |
| 4 | 10 | 3333.33 | 3.17 | 3 | 30 |
| 5 | 10 | 5000 | 2.99 | 5 | 50 |
| DMSO | 10 | 0.1 mL/10 g b.w. | - | 0 | 0 |
| Parameters | Mean ± SD |
|---|---|
| Cmax (μg/mL) | 8.69 ± 0.74 |
| Tmax (h) | 0.083 |
| T1/2 (h) | 0.22 ± 0.04 |
| Cl (L/h∙kg) | 2.76 ± 0.31 |
| MRT (h) | 0.31 ± 0.05 |
| AUC0-t (h·μg/mL) | 3.65 ± 0.40 |
| AUC0-∞ (h·μg/mL) | 3.66 ± 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, X.-Y.; Gao, H.; Gao, M.-L.; Jin, Z.; Tang, Y.-Z. Antibacterial Activity of a Promising Antibacterial Agent: 22-(4-(2-(4-Nitrophenyl-piperazin-1-yl)-acetyl)-piperazin-1-yl)-22-deoxypleuromutilin. Molecules 2021, 26, 3502. https://doi.org/10.3390/molecules26123502
Zuo X-Y, Gao H, Gao M-L, Jin Z, Tang Y-Z. Antibacterial Activity of a Promising Antibacterial Agent: 22-(4-(2-(4-Nitrophenyl-piperazin-1-yl)-acetyl)-piperazin-1-yl)-22-deoxypleuromutilin. Molecules. 2021; 26(12):3502. https://doi.org/10.3390/molecules26123502
Chicago/Turabian StyleZuo, Xiang-Yi, Hong Gao, Mei-Ling Gao, Zhen Jin, and You-Zhi Tang. 2021. "Antibacterial Activity of a Promising Antibacterial Agent: 22-(4-(2-(4-Nitrophenyl-piperazin-1-yl)-acetyl)-piperazin-1-yl)-22-deoxypleuromutilin" Molecules 26, no. 12: 3502. https://doi.org/10.3390/molecules26123502
APA StyleZuo, X.-Y., Gao, H., Gao, M.-L., Jin, Z., & Tang, Y.-Z. (2021). Antibacterial Activity of a Promising Antibacterial Agent: 22-(4-(2-(4-Nitrophenyl-piperazin-1-yl)-acetyl)-piperazin-1-yl)-22-deoxypleuromutilin. Molecules, 26(12), 3502. https://doi.org/10.3390/molecules26123502