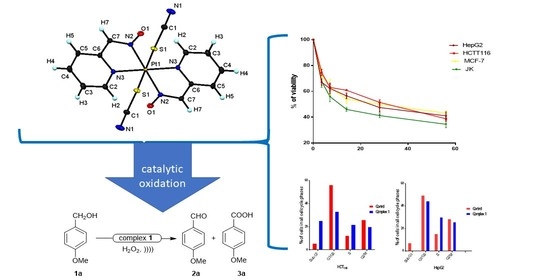
Potential Anticancer Activities and Catalytic Oxidation Efficiency of Platinum(IV) Complex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation
2.3. Synthesis of [Pt(SCN)2(paO)2] (1)
2.4. Crystallographic Studies
2.5. Assessments of IC50 of Complex 1 in HCT116, HepG2, MCF-7 Cell and JK-1 Lines by Using xMTT Assay
2.6. Determination of Apoptosis and Necrosis by Using Annexin V-FITC/Propidium Iodide (PI) in HCT116, HepG2 Treated with Complex 1
2.7. Flow Cytometry Analysis of the Cell Cycle of HCT116 and HepG2 Treated with [Pt(SCN)2(PaO)2] Using the Propidium Iodide Technique
3. Results and Discussion
3.1. Crystal Structure Description of [Pt(SCN)2(paO)2] (1)
3.2. IR Spectra
3.3. NMR Spectra
3.4. Electronic Absorption and Emission Spectra
3.5. Cytotoxicity of [Pt(SCN)2(PaO)2] Complex in Human Cancer Cell Lines
3.6. Morphology of HCT116 and HepG2 Treated with [Pt(SCN)2(PaO)2] Complex
3.7. Apoptosis and Necrosis of HCT116, HepG2 Treated with [Pt(SCN)2(PaO)2] Complex
3.8. The Cell Cycle Phases of HCT116 and HepG2 Treated with [Pt(SCN)2(PaO)2]
3.9. Catalytic Activity Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ishida, S.; Lee, J.; Thiele, D.J.; Herskowitz, I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc. Natl. Acad. Sci. USA 2002, 99, 14298–14302. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.-B. Molecular reaction mechanisms of combination treatments of low-dose cisplatin with radiotherapy and photodynamic therapy. J. Med. Chem. 2007, 50, 2601–2604. [Google Scholar] [CrossRef]
- Deo, K.M.; Ang, D.L.; McGhie, B.; Rajamanickam, A.; Dhiman, A.; Khoury, A.; Holland, J.; Bjelosevic, A.; Pages, B.; Gordon, C. Platinum coordination compounds with potent anticancer activity. Coord. Chem. Rev. 2018, 375, 148–163. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [Green Version]
- Siddik, Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Guo, Z. Targeting and delivery of platinum-based anticancer drugs. Chem. Soc. Rev. 2013, 42, 202–224. [Google Scholar] [CrossRef]
- El-bendary, M.M.; Rüffer, T.; Arshad, M.N.; Asiri, A.M. Synthesis and structure characterization of Pt(IV) and Cd(II) 1,10-phenanthroline complexes; fluorescence, antitumor and photocatalytic property. J. Mol. Struct. 2019, 1192, 230–240. [Google Scholar] [CrossRef]
- Giandomenico, C.M.; Abrams, M.J.; Murrer, B.A.; Vollano, J.F.; Rheinheimer, M.I.; Wyer, S.B.; Bossard, G.E.; Higgins, J.D. Carboxylation of kinetically inert platinum (IV) hydroxy complexes. An entr. acte. ee into orally active platinum (IV) antitumor agents. Inorg. Chem. 1995, 34, 1015–1021. [Google Scholar] [CrossRef]
- Wexselblatt, E.; Gibson, D. What do we know about the reduction of Pt (IV) pro-drugs? J. Inorg. Biochem. 2012, 117, 220–229. [Google Scholar] [CrossRef]
- Gibson, D. Multi-action Pt (IV) anticancer agents; Do we understand how they work? J. Inorg. Biochem. 2019, 191, 77–84. [Google Scholar] [CrossRef]
- Gibson, D. Pt (IV) Anticancer Prodrugs—A Tale of Mice and Men. ChemMedChem 2021, 16, 2188–2191. [Google Scholar] [CrossRef]
- Hu, W.; Fang, L.; Hua, W.; Gou, S. Biotin-Pt (IV)-indomethacin hybrid: A targeting anticancer prodrug providing enhanced cancer cellular uptake and reversing cisplatin resistance. J. Inorg. Biochem. 2017, 175, 47–57. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Q.; Huang, Z.; Yang, X.; Nie, Q.; Hao, W.; Wang, P.G.; Wang, X. Glycosylated platinum (IV) complexes as substrates for glucose transporters (GLUTs) and organic cation transporters (OCTs) exhibited cancer targeting and human serum albumin binding properties for drug delivery. J. Med. Chem. 2017, 60, 5736–5748. [Google Scholar] [CrossRef]
- Ma, J.; Yang, X.; Hao, W.; Huang, Z.; Wang, X.; Wang, P.G. Mono-functionalized glycosylated platinum (IV) complexes possessed both pH and redox dual-responsive properties: Exhibited enhanced safety and preferentially accumulated in cancer cells in vitro and in vivo. Eur. J. Med. Chem. 2017, 128, 45–55. [Google Scholar] [CrossRef]
- Muhammad, N.; Sadia, N.; Zhu, C.; Luo, C.; Guo, Z.; Wang, X. Biotin-tagged platinum (iv) complexes as targeted cytostatic agents against breast cancer cells. Chem. Commun. 2017, 53, 9971–9974. [Google Scholar] [CrossRef] [Green Version]
- El-bendary, M.M.; Saleh, T.S.; Al-Bogami, A.S. Synthesis and structural characterization of a palladium complex as an anticancer agent, and a highly efficient and reusable catalyst for the Heck coupling reaction under ultrasound irradiation: A convenient sustainable green protocol. Polyhedron 2021, 194, 114924. [Google Scholar] [CrossRef]
- Bhargava, A.; Vaishampayan, U.N. Satraplatin: Leading the new generation of oral platinum agents. Expert Opin. Investig. Drugs 2009, 18, 1787–1797. [Google Scholar] [CrossRef] [Green Version]
- Žák, F.; Turánek, J.; Kroutil, A.; Sova, P.; Mistr, A.; Poulová, A.; Mikolin, P.; Žák, Z.; Kašná, A.; Záluská, D. Platinum (IV) complex with adamantylamine as nonleaving amine group: Synthesis, characterization, and in vitro antitumor activity against a panel of cisplatin-resistant cancer cell lines. J. Med. Chem. 2004, 47, 761–763. [Google Scholar] [CrossRef]
- Ley, S.V.; Norman, J.; Griffith, W.P.; Marsden, S.P. Tetrapropylammonium perruthenate, Pr4N+ RuO4−, TPAP: A catalytic oxidant for organic synthesis. Synthesis 1994, 1994, 639–666. [Google Scholar] [CrossRef]
- Ma, Z.; Bobbitt, J.M. Organic oxoammonium salts. 3. A new convenient method for the oxidation of alcohols to aldehydes and ketones. J. Org. Chem. 1991, 56, 6110–6114. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Kochi, J.K. Metal-catalyzed oxidations of organic compounds in the liquid phase: A mechanistic approach. In Advances in Catalysis; Elsevier: Amsterdam, The Netherlands, 1976; Volume 25, pp. 272–413. [Google Scholar]
- Cainelli, G.; Cardillo, G. Chromium Oxidations in Organic Chemistry; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 19. [Google Scholar]
- Dess, D.; Martin, J. Readily accessible 12-I-5 oxidant for the conversion of primary and secondary alcohols to aldehydes and ketones. J. Org. Chem. 1983, 48, 4155–4156. [Google Scholar] [CrossRef]
- Mancuso, A.J.; Swern, D. Activated dimethyl sulfoxide: Useful reagents for synthesis. Synthesis 1981, 1981, 165–185. [Google Scholar] [CrossRef]
- Sheldon, R.; Arends, I.; Dijksman, A. New developments in catalytic alcohol oxidations for fine chemicals synthesis. Catal. Today 2000, 57, 157–166. [Google Scholar] [CrossRef]
- Xu, C.; Arancon, R.A.D.; Labidi, J.; Luque, R. Lignin depolymerisation strategies: Towards valuable chemicals and fuels. Chem. Soc. Rev. 2014, 43, 7485–7500. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.; Bourhis, L.; Gildea, R.; Howard, J.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Ali, E.M.; Elashkar, A.A.; El-Kassas, H.Y.; Salim, E.I. Methotrexate loaded on magnetite iron nanoparticles coated with chitosan: Biosynthesis, characterization, and impact on human breast cancer MCF-7 cell line. Int. J. Biol. Macromol. 2018, 120, 1170–1180. [Google Scholar] [CrossRef]
- Plumb, J.A. Cell sensitivity assays: The MTT assay. In Cancer Cell Culture; Springer: Berlin/Heidelberg, Germany, 2004; pp. 165–169. [Google Scholar]
- Achiron, A.; Feldman, A.; Karmona, L.; Pe’er, L.; Avizemer, H.; Bartov, E.; Burgansky, Z.; Rosner, M.; Vishnevskia-Dai, V. Effect of Rho-associated kinase inhibitor on human corneal endothelial cell apoptosis. J. Cataract. Refract. Surg. 2020, 46, 612–616. [Google Scholar] [CrossRef]
- Fried, J.; Perez, A.G.; Clarkson, B.D. Flow cytofluorometric analysis of cell cycle distributions using propidium iodide. Properties of the method and mathematical analysis of the data. J. Cell Biol. 1976, 71, 172–181. [Google Scholar] [CrossRef]
- Alinaghi, M.; Karami, K.; Shahpiri, A.; Nasab, A.K.; Momtazi-Borojeni, A.A.; Abdollahi, E.; Lipkowski, J. A Pd(II) complex derived from pyridine-2-carbaldehyde oxime ligand: Synthesis, characterization, DNA and BSA interaction studies and in vitro anticancer activity. J. Mol. Struct. 2020, 1219, 128479. [Google Scholar] [CrossRef]
- Ozawa, Y.; Kima, M.; Toriumi, K. A one-dimensional platinum mixedvalence complex with bridging thiocyanate S atoms: [[PtII(en)2](μ-SCN)-[PtIV(en)2](μ-SCN)](ClO4)4 (en is ethane-1,2-diamine. Acta Crystallogr. 2013, C69, 146–149. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Jaffé, H.H.; Orchin, M. Theory and applications of ultraviolet spectroscopy. J. Am. Chem. Soc. 1963, 85, 4056–4057. [Google Scholar] [CrossRef]
- Etaiw, S.E.-d.H.; El-bendary, M.M. Metal-organic framework constructed by copper (i) cyanide and ethyl isonicotinate through hydrogen bonding. J. Inorg. Organomet. Polym. Mater. 2010, 20, 739–745. [Google Scholar] [CrossRef]
- Wong, W.-Y.; Ho, C.-L. Heavy metal organometallic electrophosphors derived from multi-component chromophores. Coord. Chem. Rev. 2009, 253, 1709–1758. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, G.; Wong, W.-Y. Functionalization of phosphorescent emitters and their host materials by main-group elements for phosphorescent organic light-emitting devices. Chem. Soc. Rev. 2015, 44, 8484–8575. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, M.; Wang, H.; Fang, L.; Gou, S. Targeting RAS-RAF pathway significantly improves antitumor activity of Rigosertib-derived platinum (IV) complexes and overcomes cisplatin resistance. Eur. J. Med. Chem. 2020, 194, 112269. [Google Scholar] [CrossRef]
- Todd, R.C.; Lippard, S.J. Inhibition of transcription by platinum antitumor compounds. Metallomics 2009, 1, 280–291. [Google Scholar] [CrossRef] [Green Version]
- Momčilović, O.; Navara, C.; Schatten, G. Cell cycle adaptations and maintenance of genomic integrity in embryonic stem cells and induced pluripotent stem cells. Cell Cycle Dev. 2011, 53, 415–458. [Google Scholar]
- Suslick, K.; Fang, M.; Hyeon, T.; Mdleleni, M. Applications of sonochemistry to materials synthesis. In Sonochemistry and Sonoluminescence; Springer: Berlin/Heidelberg, Germany, 1999; pp. 291–320. [Google Scholar]
- Kaur, S.; Singh, V. Visible light induced sonophotocatalytic degradation of Reactive Red dye 198 using dye sensitized TiO2. Ultrason. Sonochemistry 2007, 14, 531–537. [Google Scholar] [CrossRef]
- Shirgaonkar, I.Z.; Pandit, A.B. Sonophotochemical destruction of aqueous solution of 2, 4, 6-trichlorophenol. Ultrason. Sonochemistry 1998, 5, 53–61. [Google Scholar] [CrossRef]
- Missaghi, M.N.; Galloway, J.M.; Kung, H.H. Bis (pyridyl) siloxane-Pd (II) complex catalyzed oxidation of alcohol to aldehyde: Effect of ligand tethering on catalytic activity and deactivation behavior. Appl. Catal. A Gen. 2011, 391, 297–304. [Google Scholar] [CrossRef]
- Racles, C.; Zaltariov, M.F.; Damoc, M.; Macsim, A.M.; Iacob, M.; Sacarescu, L. Three Reactions, One Catalyst: A Multi-Purpose Platinum (IV) Complex and its Silica-Supported Homologue for Environmentally Friendly Processes. Appl. Organomet. Chem. 2020, 34, e5422. [Google Scholar] [CrossRef]
- Tonucci, L.; Nicastro, M.; d’Alessandro, N.; Bressan, M.; D’Ambrosio, P.; Morvillo, A. Catalytic aerobic oxidation of allylic alcohols to carbonyl compounds under mild conditions. Green Chem. 2009, 11, 816–820. [Google Scholar] [CrossRef]
- Ashby, M.T. Hypothiocyanite. In Advances in Inorganic Chemistry; Elsevier: Amsterdam, The Netherlands, 2012; Volume 64, pp. 263–303. [Google Scholar]















| Compound | PaOH (L) | Complex 1 |
|---|---|---|
| ν(OH) | 3186 m | - |
| ν(CH)(arom) | 3077 w, 3005 w | 3048 w |
| ν(CH)(aliph) | 2884 w | 2925 m |
| ν(C≡N) | - | 2126 s |
| ν(C=N),ν(C=C) | 1592 s, 1514 s, 1472 m | 1607 s, 1512 s, 1485 m |
| Skeletal and C-C vibrs. of L | 1321 m, 1295 w, 1155 m, 1099 m | 1358 m, 1253 s, 1115 m |
| δCHof L | 1434 | 1429 s |
| γCHof L | 767 s | 769 s |
| ν(NO) | 976 s | 1049 s |
| ν(Pt-S) | - | 567 w |
| Absorption Spectrum (λabs (nm)) | Emission Spectrum (λem (nm)) | |||
|---|---|---|---|---|
| paOH | Complex 1 | assignment | Complex 1 | Assignment |
| 220 | 208 | 1La←1A | 355 | π-π* transition |
| 265 | 230 | 1Lb←1A | 415 | MLCT transitions |
| 315 | - 278 | n–π* π*–π* | ||
| Type of Cells | HepG2 | HCTT116 | MCF-7 | JK-1 |
|---|---|---|---|---|
| IC50 (Range) | 14–25 | 18–28 | 16–27 | 10–17 |
| IC50 (Value) | 19 ± 6 | 21 ± 5 | 22 ± 6 | 13 ± 3 |
| HCTT116 | HepG2 | |||
|---|---|---|---|---|
| Control | Complex 1 | Control | Complex 1 | |
| % of viable cells (Lower left) | 84.4 | 23.5 | 85.8 | 80.2 |
| % of necrosis (Upper left) | 5.1 | 27.8 | 14 | 19.7 |
| % of Early apoptosis (Lower right) | 1.8 | 10.5 | 0.2 | 0.1 |
| % of Late apoptosis (Upper right) | 8.7 | 23.5 | 0 | 0 |
| Total % of apoptosis | 10.5 | 34 | 0.2 | 0.1 |
| Cell Phase | HCTT116 | HepG2 | ||
|---|---|---|---|---|
| Control | Complex 1 | Control | Complex 1 | |
| Sub G1 | 5.5 | 25 | 6.9 | 0 |
| G1/G0 | 56.4 | 33 | 49.2 | 44.4 |
| S | 12.3 | 21.7 | 15.3 | 29.8 |
| G2/M | 25.8 | 20 | 28.5 | 25.8 |
| Entry | Catalyst Amount (mol%) | Molar Ratio. (Substrate: Oxidant) | Temp. °C | Solvent | Ultrasonics Irrad. | ||
|---|---|---|---|---|---|---|---|
| Yield% | Time (min.) | ||||||
| 2a | 3a | ||||||
| 1 | 0 | 1:1.5 | 30–35 | Solventless | 0 | 0 | 180 |
| 2 | 5 | 1:1.5 | 30–35 | Solventless | 57 | 0 | 90 |
| 3 | 10 | 1:1.5 | 30–35 | Solventless | 93 | 0 | 30 |
| 4 | 10 | 1:1.5 | 60–70 | Solventless | 96 | 0 | 30 |
| 5 | 10 | 1:1.5 | 75–80 | Solventless | 96 | 0 | 30 |
| 6 | 10 | 1:2 | 60–70 | Solventless | 96 | 0 | 30 |
| 7 | 15 | 1:1.5 | 60–70 | Solventless | 90 | 4 | 60 |
| 8 | 10 | 1:1 | 60–70 | Solventless | 93 | 0 | 60 |
| 9 | 10 | 1:1.5 | 60–70 | Water | 90 | 0 | 60 |
| 10 | 10 | 1:1.5 | 60–70 | 1,4-dioxan | 40 | 0 | 120 |
| 11 | 10 | 1:1.5 | 60–70 | Acetonitrile | 47 | 0 | 120 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-bendary, M.M.; Saleh, T.S.; Alomari, M.M.; Ali, E.M.M.; Davaasuren, B.; Jaremko, M.; Babgi, B.A. Potential Anticancer Activities and Catalytic Oxidation Efficiency of Platinum(IV) Complex. Molecules 2022, 27, 4406. https://doi.org/10.3390/molecules27144406
El-bendary MM, Saleh TS, Alomari MM, Ali EMM, Davaasuren B, Jaremko M, Babgi BA. Potential Anticancer Activities and Catalytic Oxidation Efficiency of Platinum(IV) Complex. Molecules. 2022; 27(14):4406. https://doi.org/10.3390/molecules27144406
Chicago/Turabian StyleEl-bendary, Mohamed M., Tamer S. Saleh, Mansour M. Alomari, Ehab M. M. Ali, Bambar Davaasuren, Mariusz Jaremko, and Bandar A. Babgi. 2022. "Potential Anticancer Activities and Catalytic Oxidation Efficiency of Platinum(IV) Complex" Molecules 27, no. 14: 4406. https://doi.org/10.3390/molecules27144406
APA StyleEl-bendary, M. M., Saleh, T. S., Alomari, M. M., Ali, E. M. M., Davaasuren, B., Jaremko, M., & Babgi, B. A. (2022). Potential Anticancer Activities and Catalytic Oxidation Efficiency of Platinum(IV) Complex. Molecules, 27(14), 4406. https://doi.org/10.3390/molecules27144406