Composition of Pinot Noir Wine from Grapevine Red Blotch Disease-Infected Vines Managed with Exogenous Abscisic Acid Applications
Abstract
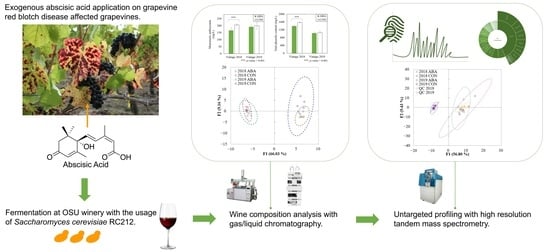
:1. Introduction
2. Results
2.1. Effects of ABA Vine Treatment on Anthocyanin and Phenolics of Grapevine Red Blotch Disease-Associated Wines
2.2. Volatile Compounds
2.3. Untargeted LC-MS/MS Fingerprinting
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Field Experiment
4.3. Winemaking
4.4. Wine Monomeric Anthocyanin
4.5. Total Phenolic Content
4.6. Major Phenolic Composition
4.7. Wine Volatile Analysis
4.8. Wine C13-Norisoprenoids (Bound-Form) Analysis
4.9. Untargeted Analysis with Liquid Chromatography High-Resolution Tandem Mass Spectrometry (LC-HRMS/MS)
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Xiao, H.; Shabanian, M.; Moore, C.; Li, C.; Meng, B. Survey for major viruses in commercial Vitis vinifera wine grapes in Ontario. Virol. J. 2018, 15, 127. [Google Scholar] [CrossRef] [PubMed]
- Marwal, A.; Kumar, R.; Paul Khurana, S.M.; Gaur, R.K. Complete nucleotide sequence of a new geminivirus isolated from Vitis vinifera in India: A symptomless host of Grapevine red blotch virus. Virusdisease 2019, 30, 106–111. [Google Scholar] [CrossRef]
- Reynard, J.S.; Brodard, J.; Dubuis, N.; Zufferey, V.; Schumpp, O.; Schaerer, S.; Gugerli, P. Grapevine red blotch virus: Absence in Swiss Vineyards and Analysis of Potential Detrimental Effect on Viticultural Performance. Plant Dis. 2018, 102, 651–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.; Igori, D.; Zhao, F.; Moon, J.S.; Cho, L.S.; Choi, G.S. First Report of Grapevine red blotch-associated virus on Grapevine in Korea. Plant Dis. 2016, 100, 1957. [Google Scholar] [CrossRef]
- Gasperin-Bulbarela, J.; Licea-Navarro, A.F.; Pino-Villar, C.; Hernandez-Martinez, R.; Carrillo-Tripp, J. First Report of Grapevine Red Blotch Virus in Mexico. Plant Dis. 2019, 103, 381. [Google Scholar] [CrossRef]
- Luna, F.; Debat, H.; Moyano, S.; Zavallo, D.; Asurmendi, S.; Gomez-Talquenca, S. First report of grapevine red blotch virus infecting grapevine in Argentina. J. Plant Pathol. 2019, 101, 1239. [Google Scholar] [CrossRef] [Green Version]
- Krenz, B.; Thompson, J.R.; McLane, H.L.; Fuchs, M.; Perry, K.L. Grapevine red blotch-associated virus Is Widespread in the United States. Phytopathology 2014, 104, 1232–1240. [Google Scholar] [CrossRef] [Green Version]
- Sudarshana, M.R.; Perry, K.L.; Fuchs, M.F. Grapevine Red Blotch-Associated Virus, an Emerging Threat to the Grapevine Industry. Phytopathology 2015, 105, 1026–1032. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Luscher, J.; Plank, C.M.; Brillante, L.; Cooper, M.L.; Smith, R.J.; Al-Rwahnih, M.; Yu, R.; Oberholster, A.; Girardello, R.; Kurtural, S.K. Grapevine Red Blotch Virus May Reduce Carbon Translocation Leading to Impaired Grape Berry Ripening. J. Agric. Food Chem. 2019, 67, 2437–2448. [Google Scholar] [CrossRef]
- Cauduro Girardello, R.; Rich, V.; Smith, R.J.; Brenneman, C.; Heymann, H.; Oberholster, A. The impact of grapevine red blotch disease on Vitis vinifera L. Chardonnay grape and wine composition and sensory attributes over three seasons. J. Sci. Food Agric. 2020, 100, 1436–1447. [Google Scholar] [CrossRef]
- Blanco-Ulate, B.; Hopfer, H.; Figueroa-Balderas, R.; Ye, Z.; Rivero, R.M.; Albacete, A.; Perez-Alfocea, F.; Koyama, R.; Anderson, M.M.; Smith, R.J.; et al. Red blotch disease alters grape berry development and metabolism by interfering with the transcriptional and hormonal regulation of ripening. J. Exp. Bot. 2017, 68, 1225–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, G.E.; Padhi, E.M.; Sudarshana, M.R.; Fialho, F.B.; Medina-Plaza, C.; Girardello, R.C.; Tseng, D.; Bruce, R.C.; Erdmann, J.N.; Slupsky, C.M. Impact of grapevine red blotch disease on primary and secondary metabolites in ‘Cabernet Sauvignon’grape tissues. Food Chem. 2021, 342, 128312. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.D.; Kc, A.N. Water Deficits Do Not Improve Fruit Quality in Grapevine Red Blotch Virus-Infected Grapevines (Vitis vinifera L.). Front. Plant Sci. 2020, 11, 1292. [Google Scholar] [CrossRef] [PubMed]
- Bucchetti, B.; Matthews, M.A.; Falginella, L.; Peterlunger, E.; Castellarin, S.D. Effect of water deficit on Merlot grape tannins and anthocyanins across four seasons. Sci. Hortic. 2011, 128, 297–305. [Google Scholar] [CrossRef]
- Girardello, R.C.; Cooper, M.L.; Smith, R.J.; Lerno, L.A.; Bruce, R.C.; Eridon, S.; Oberholster, A. Impact of Grapevine Red Blotch Disease on Grape Composition of Vitis vinifera Cabernet Sauvignon, Merlot, and Chardonnay. J. Agric. Food Chem. 2019, 67, 5496–5511. [Google Scholar] [CrossRef]
- Fenoll, J.; Manso, A.; Hellín, P.; Ruiz, L.; Flores, P. Changes in the aromatic composition of the Vitis vinifera grape Muscat Hamburg during ripening. Food Chem. 2009, 114, 420–428. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic Acid Signaling and Abiotic Stress Tolerance in Plants: A Review on Current Knowledge and Future Prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef] [Green Version]
- Miliordos, D.-E.; Tsiknia, M.; Kontoudakis, N.; Dimopoulou, M.; Bouyioukos, C.; Kotseridis, Y. Impact of Application of Abscisic Acid, Benzothiadiazole and Chitosan on Berry Quality Characteristics and Plant Associated Microbial Communities of Vitis vinifera L var. Mouhtaro Plants. Sustainability 2021, 13, 5802. [Google Scholar] [CrossRef]
- Hatmi, S.; Villaume, S.; Trotel-Aziz, P.; Barka, E.A.; Clement, C.; Aziz, A. Osmotic Stress and ABA Affect Immune Response and Susceptibility of Grapevine Berries to Gray Mold by Priming Polyamine Accumulation. Front. Plant Sci. 2018, 9, 1010. [Google Scholar] [CrossRef] [Green Version]
- Alenazi, M.M.; Shafiq, M.; Alobeed, R.S.; Alsdon, A.A.; Abbasi, N.A.; Ali, I.; Mubushar, M.; Javed, I. Application of abscisic acid at veraison improves red pigmentation and accumulation of dietary antioxidants in red table grapes cv. Red Globe at harvest. Sci. Hortic. 2019, 257, 9. [Google Scholar] [CrossRef]
- Alonso, R.; Berli, F.J.; Fontana, A.; Piccoli, P.; Bottini, R. Abscisic Acid’s Role in the Modulation of Compounds that Contribute to Wine Quality. Plants 2021, 10, 938. [Google Scholar] [CrossRef] [PubMed]
- Katayama-Ikegami, A.; Sakamoto, T.; Shibuya, K.; Katayama, T.; Gao-Takai, M. Effects of abscisic acid treatment on berry coloration and expression of flavonoid biosynthesis genes in grape. Am. J. Plant Sci. 2016, 7, 1325. [Google Scholar] [CrossRef] [Green Version]
- Monagas, M.; Bartolomé, B.; Gómez-Cordovés, C. Updated knowledge about the presence of phenolic compounds in wine. Crit. Rev. Food Sci. Nutr. 2005, 45, 85–118. [Google Scholar] [CrossRef] [PubMed]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef]
- Sparvoli, F.; Martin, C.; Scienza, A.; Gavazzi, G.; Tonelli, C. Cloning and molecular analysis of structural genes involved in flavonoid and stilbene biosynthesis in grape (Vitis vinifera L.). Plant Mol. Biol. 1994, 24, 743–755. [Google Scholar] [CrossRef]
- Arbulu, M.; Sampedro, M.; Gómez-Caballero, A.; Goicolea, M.; Barrio, R. Untargeted metabolomic analysis using liquid chromatography quadrupole time-of-flight mass spectrometry for non-volatile profiling of wines. Anal. Chim. Acta 2015, 858, 32–41. [Google Scholar] [CrossRef]
- Flamini, R.; De Rosso, M. Chapter 5—High-Resolution Mass Spectrometry and Biological Properties of Grapevine and Wine Stilbenoids. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 61, pp. 175–210. [Google Scholar]
- Villamor, R.R.; Ross, C.F. Wine Matrix Compounds Affect Perception of Wine Aromas. Annu. Rev. Food Sci. Technol. 2013, 4, 1–20. [Google Scholar] [CrossRef]
- Gonzalezbarreiro, C.; Rialotero, R.; Canchogrande, B.; Simalgandara, J. Wine Aroma Compounds in Grapes: A Critical Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218. [Google Scholar] [CrossRef]
- Kanasawud, P.; Crouzet, J.C. Mechanism of formation of volatile compounds by thermal degradation of carotenoids in aqueous medium. 1. beta.-Carotene degradation. J. Agric. Food Chem. 1990, 38, 237–243. [Google Scholar] [CrossRef]
- Williams, P.J.; Sefton, M.A.; Francis, I.L. Glycosidic precursors of varietal grape and wine flavor. ACS Symp. Ser. 1992, 490, 74–86. [Google Scholar]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Bell, S.J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Liu, S.; Ma, D.; Li, Z.; Sun, H.; Mao, J.; Shi, Y.; Han, X.; Zhou, Z.; Mao, J. Assimilable nitrogen reduces the higher alcohols content of huangjiu. Food Control 2021, 121, 107660. [Google Scholar] [CrossRef]
- Hoffman, S. Use of Organoleptic Methodologies to Evaluate Wine Oligosaccharides and Wines made from Grapevine Red Blotch Virus Positive Vines Subjected to Various Vineyard Management Practices. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 2021. [Google Scholar]
- Fang, Y.; Qian, M.C. Development of C6 and other volatile compounds in Pinot Noir grapes determined by Stir Bar Sorptive Extraction-GC-MS. In Flavor Chemistry of Wine and Other Alcoholic Beverages; ACS Publications: Washington, DC, USA, 2012; pp. 81–99. [Google Scholar]
- Chong, Z.; Hao, C.; Lianjun, W. Effect of ABA treatment on the expression of ADH gene family and C6 volatile production in table grape (V. vinifera cv. Muscat Hamburg) during postharvest storage. Acta Physiol. Plant. 2020, 42, 1–8. [Google Scholar] [CrossRef]
- He, L.; Meng, N.; Castellarin, S.D.; Wang, Y.; Sun, Q.; Li, X.-Y.; Dong, Z.-G.; Tang, X.-P.; Duan, C.-Q.; Pan, Q.-H. Combined Metabolite and Transcriptome Profiling Reveals the Norisoprenoid Responses in Grape Berries to Abscisic Acid and Synthetic Auxin. Int. J. Mol. Sci. 2021, 22, 1420. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Qian, M.C. Development of C13-norisoprenoids, carotenoids and other volatile compounds in Vitis vinifera L. Cv. Pinot noir grapes. Food Chem. 2016, 192, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.G. Managing Wine Quality: Viticulture and Wine Quality; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Yuan, F.; Schreiner, R.P.; Osborne, J.; Qian, M.C. Effects of Soil NPK Supply on Pinot noir Wine Phenolics and Aroma Composition. Am. J. Enol. Vitic. 2018, 69, 371–385. [Google Scholar] [CrossRef]
- Feng, H.; Skinkis, P.A.; Qian, M.C. Pinot noir wine volatile and anthocyanin composition under different levels of vine fruit zone leaf removal. Food Chem. 2017, 214, 736–744. [Google Scholar] [CrossRef]
- Alcazar Magana, A.; Wright, K.; Vaswani, A.; Caruso, M.; Reed, R.L.; Bailey, C.F.; Nguyen, T.; Gray, N.E.; Soumyanath, A.; Quinn, J.; et al. Integration of mass spectral fingerprinting analysis with precursor ion (MS1) quantification for the characterisation of botanical extracts: Application to extracts of Centella asiatica (L.) Urban. Phytochem. Anal. 2020, 31, 722–738. [Google Scholar] [CrossRef] [Green Version]
- Yap, E.S.P.; Uthairatanakij, A.; Laohakunjit, N.; Jitareerat, P.; Vaswani, A.; Magana, A.A.; Morre, J.; Maier, C.S. Plant growth and metabolic changes in ‘Super Hot’ chili fruit (Capsicum annuum) exposed to supplemental LED lights. Plant Sci. 2021, 305, 110826. [Google Scholar] [CrossRef]
- Bading-Taika, B.; Akinyeke, T.; Magana, A.A.; Choi, J.; Ouanesisouk, M.; Torres, E.R.S.; Lione, L.A.; Maier, C.S.; Bobe, G.; Raber, J.; et al. Phytochemical characterization of Tabernanthe iboga root bark and its effects on dysfunctional metabolism and cognitive performance in high-fat-fed C57BL/6J mice. J. Food Bioact. 2018, 3, 111–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, S.F.; Chevallier, O.P.; Elliott, C.T.; Holscher, C.; Johnston, J.; McGuinness, B.; Kehoe, P.G.; Passmore, A.P.; Green, B.D. Untargeted metabolomic analysis of human plasma indicates differentially affected polyamine and L-arginine metabolism in mild cognitive impairment subjects converting to Alzheimer’s disease. PLoS ONE 2015, 10, e0119452. [Google Scholar] [CrossRef] [PubMed]





| Compound 1 | Vintage 2018 | Vintage 2019 | ||||
|---|---|---|---|---|---|---|
| ABA | CON | p-Value 2 | ABA | CON | p-Value 2 | |
| caffeic acid | 3.8 ± 0.3 | 4.2 ± 0.4 | 0.214 | 4.1 ± 1.4 | 5.0 ± 0.8 | 0.598 |
| caffeoyltartaric acid | 20.3 ± 0.7 a 3 | 23.6 ± 0.5 b | < 0.001 | 9.7 ± 5.4 | 13.1 ± 3.1 | 0.219 |
| catechin | 67.0 ± 6.0 | 71.8 ± 6.2 | 0.093 | 34.3 ± 2.6 | 35.3 ± 3.3 | 0.183 |
| epicatechin | 83.6 ± 7.5 | 81.0 ± 5.0 | 0.500 | 91.4 ± 15.0 | 83.5 ± 25.4 | 0.526 |
| fertaric acid | 7.2 ± 1.3 | 7.3 ± 1.3 | 0.901 | 1.7 ± 0.1 | 1.6 ± 0.1 | 0.322 |
| gallic acid | 9.4 ± 0.8 | 9.3 ± 0.5 | 0.741 | 6.5 ± 0.5 | 6.8 ± 0.4 | 0.275 |
| malvidin-3-o-glucoside | 124 ± 2 a | 141 ± 10 b | 0.002 | 263 ± 14 | 266 ± 21 | 0.732 |
| p-coumaric acid | 3.9 ± 0.4 | 3.8 ± 0.8 | 0.899 | 4.3 ± 1.1 | 4.9 ± 1.0 | 0.389 |
| resveratrol | <1 | <1 | <1 | <1 | ||
| trans-coutaric acid | 8.3 ± 1.2 | 8.0 ± 0.3 | 0.517 | 9.6 ± 0.7 a | 10.8 ± 0.5 b | 0.009 |
| vanillic acid | 10.1 ± 1 | 11.4 ± 1.5 | 0.127 | <1 | <1 | |
| Compound 1 | Vintage 2018 | Vintage 2019 | ||||
|---|---|---|---|---|---|---|
| ABA | CON | p-Value 2 | ABA | CON | p-Value 2 | |
| acid | ||||||
| 3-methylbutanoic acid 3 | nd 4 | nd | 1.2 ± 0.1 a 5 | 1.4 ± 0.1 b | 0.044 | |
| decanoic acid | 269 ± 12 | 251 ± 18 | 0.068 | 141 ± 8 | 143 ± 8 | 0.680 |
| hexanoic acid | 395 ± 21 | 400 ± 8 | 0.580 | 1226 ± 41 a | 1352 ± 113 b | 0.028 |
| octanoic acid | 902 ± 35 | 890 ± 49 | 0.653 | 772 ± 28 | 837 ± 73 | 0.070 |
| total | 1566 | 1541 | 3339 | 3732 | ||
| alcohol | ||||||
| (E)-2-hexen-1-ol | 47.4 ± 12.8 | 58.5 ± 11.5 | 0.146 | 624 ± 109 | 650 ± 151 | 0.478 |
| (E)-3-hexen-1-ol | 135 ± 17 a | 111 ± 11 b | 0.013 | 326 ± 40 | 304 ± 58 | 0.752 |
| (Z)-3-hexen-1-ol | 31.7 ± 9.3 | 22.1 ± 7.5 | 0.080 | 52.4 ± 9.5 | 47.8 ± 10.0 | 0.496 |
| 1-hexanol 3 | 3.1 ± 0.3 a | 2.5 ± 0.2 b | 0.004 | 8.8 ± 1.2 | 10.6 ± 2.3 | 0.147 |
| 1-octen-3-ol | 3.6 ± 0.4 | 3.2 ± 0.9 | 0.415 | 4.2 ± 0.4 | 4.3 ± 0.4 | 0.670 |
| 2-heptanol | 6.8 ± 0.9 a | 4.7 ± 0.7 b | 0.001 | 11.7 ± 3.0 | 12.6 ± 1.5 | 0.534 |
| benzyl alcohol | 947 ± 34 a | 890 ± 41 b | 0.026 | 825 ± 43 | 780 ± 73 | 0.218 |
| isoamyl alcohol 3 | 341 ± 24 a | 378 ± 11 b | 0.006 | 123 ± 12 | 128 ± 21 | 0.703 |
| isobutyl alcohol 3 | 144 ± 20 | 159 ± 12 | 0.132 | 310 ± 24 | 316 ± 27 | 0.644 |
| 2-phenylethanol 3 | 39.5 ± 1.7 a | 41.9 ± 1.7 b | 0.036 | 27.5 ± 1.0 a | 35.0 ± 6.7 b | 0.022 |
| propanol 3 | 23.0 ± 1.9 | 21.9 ± 2.8 | 0.472 | 37.0 ± 2.6 a | 32.5 ± 2.2 b | 0.009 |
| total | 551,772 | 604,390 | 508,143 | 523,899 | ||
| aldehyde and ketone | ||||||
| 6-methyl-5-hepten-2-one | nd | nd | 2.2 ± 0.3 | 2.3 ± 0.8 | 0.688 | |
| acetaldehyde 3 | 11.5 ± 1.6 | 11.9 ± 0.5 | 0.561 | nd | nd | |
| total | 11,500 | 11,900 | 2.2 | 2.3 | ||
| C13-norisoprenoid (free-form) | ||||||
| vitispirane 6 | nd | nd | 2.5 ± 0.1 | 2.8 ± 0.5 | 0.185 | |
| β-damascenone | 5.7 ± 0.2 a | 5.4 ± 0.2 b | 0.024 | 5.2 ± 0.2 | 5.8 ± 0.9 | 0.135 |
| β-ionone | 0.6 ± 0.0 a | 0.5 ± 0.0 b | 0.015 | 3.0 ± 0.0 | 3.1 ± 0.1 | 0.351 |
| total | 6.3 | 5.9 | 10.7 | 11.7 | ||
| C13-norisoprenoid (bound-form) | ||||||
| TDN 7 | 10.6 ± 0.8 a | 14.8 ± 2.3 b | 0.002 | 31.6 ± 3.2 | 31.5 ± 4.4 | 0.959 |
| vitispirane 6 | 32.6 ± 4.0 a | 41.3 ± 4.2 b | 0.004 | 101 ± 7 | 105 ± 13 | 0.563 |
| β-damascenone | 16.2 ± 1.6 | 15.9 ± 2.2 | 0.764 | 61.8 ± 4.2 | 60.2 ± 8.1 | 0.666 |
| β-ionone | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.111 | 2.5 ± 0.5 | 3.1 ± 0.7 | 0.134 |
| total | 60 | 73 | 197 | 200 | ||
| ester | ||||||
| ethyl 2-methylbutanoate | 5.0 ± 0.2 | 5.1 ± 0.4 | 0.500 | 3.9 ± 0.6 a | 5.1 ± 0.3 b | 0.001 |
| ethyl 2-methylpropanoate | 97.6 ± 10.1 | 100.4 ± 8.7 | 0.621 | 62.6 ± 5.4 a | 73.2 ± 8.2 b | 0.024 |
| ethyl 3-methylbutanoate | 6.4 ± 0.3 | 6.4 ± 0.3 | 0.755 | 5.7 ± 0.5 a | 6.6 ± 0.6 b | 0.027 |
| ethyl acetate 3 | 57.4 ± 2.5 | 54.4 ± 3.8 | 0.135 | 32.1 ± 0.8 | 32.4 ± 1.3 | 0.618 |
| ethyl butanoate | 72.0 ± 4.5 | 69.9 ± 5.3 | 0.471 | 84.6 ± 3.2 | 92.7 ± 8.9 | 0.064 |
| ethyl decanoate | 27.1 ± 2.5 a | 24.0 ± 1.5 b | 0.027 | 35.6 ± 2.5 a | 29.5 ± 2.6 b | 0.002 |
| ethyl dodecanoate | 27.9 ± 6.6 a | 20.0 ± 3.8 b | 0.030 | 12.6 ± 1.1 | 13.0 ± 3.1 | 0.811 |
| ethyl hexanoate | 183 ± 9 | 165 ± 21 | 0.086 | 235 ± 15 | 219 ± 14 | 0.078 |
| ethyl octanoate | 55.3 ± 7.6 | 56.1 ± 7.2 | 0.847 | 72.9 ± 2.6 a | 81.3 ± 7.3 b | 0.028 |
| ethyl phenylacetate | 4.2 ± 0.7 | 5.0 ± 0.8 | 0.101 | 1.1 ± 0.1 | 1.2 ± 0.1 | 0.081 |
| ethyl propionate | 75.0 ± 8.5 | 76.5 ± 7.2 | 0.753 | 95.7 ± 4.9 a | 108.6 ± 9.9 b | 0.017 |
| ethyl undecanoate | nd | nd | 5.3 ± 0.1 a | 5.0 ± 0.3 b | 0.035 | |
| hexyl acetate | 6.4 ± 1.0 | 6.0 ± 1.0 | 0.490 | 9.0 ± 0.4 | 8.6 ± 1.8 | 0.535 |
| isoamyl acetate | 386 ± 62 | 364 ± 27 | 0.430 | 391 ± 9 a | 481 ± 67 b | 0.009 |
| isobutyl acetate | 5.3 ± 0.2 | 5.2 ± 0.3 | 0.557 | 61.1 ± 2.4 a | 71.6 ± 9.9 b | 0.031 |
| phenethyl acetate | 25.7 ± 3.8 | 27.3 ± 2.5 | 0.404 | 20.1 ± 0.5 a | 21.8 ± 1.6 b | 0.039 |
| total | 58,377 | 55,331 | 33,196 | 33,618 | ||
| lactone | ||||||
| γ-decalactone | 1.6 ± 0.1 | 1.6 ± 0.1 | 0.181 | nd | nd | |
| δ-undecalactone | 2.7 ± 0.4 | 2.6 ± 0.2 | 0.539 | nd | nd | |
| total | 4.3 | 4.2 | 0 | 0 | ||
| methoxypyrazine | ||||||
| IBMP 8 | 2.7 ± 0.7 | 2.6 ± 0.9 | 0.745 | 2.3 ± 0.3 a | 2.9 ± 0.3 b | 0.013 |
| IPMP 9 | 2.4 ± 0.5 | 2.7 ± 0.2 | 0.207 | 2.0 ± 0.3 | 2.2 ± 0.6 | 0.488 |
| SBMP 10 | 15.5 ± 2.2 | 15.9 ± 1.8 | 0.781 | 22.9 ± 1.5 | 20.9 ± 4.5 | 0.334 |
| total 11 | 21 | 21 | 27 | 26 | ||
| terpene | ||||||
| citronellol | 11.6 ± 0.7 | 11.6 ± 0.7 | 0.940 | 25.7 ± 0.5 | 25.5 ± 0.7 | 0.532 |
| geraniol | 26.9 ± 0.6 | 26.4 ± 1.1 | 0.346 | 2.9 ± 0.4 | 3.6 ± 1.0 | 0.159 |
| linalool | 8.4 ± 0.3 a | 9.6 ± 0.8 b | 0.006 | 7.7 ± 0.2 | 9.3 ± 2.1 | 0.084 |
| nerol | 9.1 ± 2.9 a | 5.1 ± 2.1 b | 0.020 | 3.0 ± 0.1 | 3.8 ± 1.1 | 0.129 |
| α-terpinol | 3.8 ± 0.2 | 4.0 ± 0.6 | 0.524 | 4.5 ± 0.6 | 4.9 ± 1.1 | 0.399 |
| total | 60 | 57 | 44 | 47 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Alcazar Magana, A.; Skinkis, P.A.; Osborne, J.; Qian, Y.L.; Qian, M.C. Composition of Pinot Noir Wine from Grapevine Red Blotch Disease-Infected Vines Managed with Exogenous Abscisic Acid Applications. Molecules 2022, 27, 4520. https://doi.org/10.3390/molecules27144520
Huang L, Alcazar Magana A, Skinkis PA, Osborne J, Qian YL, Qian MC. Composition of Pinot Noir Wine from Grapevine Red Blotch Disease-Infected Vines Managed with Exogenous Abscisic Acid Applications. Molecules. 2022; 27(14):4520. https://doi.org/10.3390/molecules27144520
Chicago/Turabian StyleHuang, Ling, Armando Alcazar Magana, Patricia A. Skinkis, James Osborne, Yanping L. Qian, and Michael C. Qian. 2022. "Composition of Pinot Noir Wine from Grapevine Red Blotch Disease-Infected Vines Managed with Exogenous Abscisic Acid Applications" Molecules 27, no. 14: 4520. https://doi.org/10.3390/molecules27144520
APA StyleHuang, L., Alcazar Magana, A., Skinkis, P. A., Osborne, J., Qian, Y. L., & Qian, M. C. (2022). Composition of Pinot Noir Wine from Grapevine Red Blotch Disease-Infected Vines Managed with Exogenous Abscisic Acid Applications. Molecules, 27(14), 4520. https://doi.org/10.3390/molecules27144520