Paramagnetic Properties and Moderately RapidConformational Dynamics in the Cobalt(II) Calix[4]arene Complex by NMR
Abstract
:1. Introduction
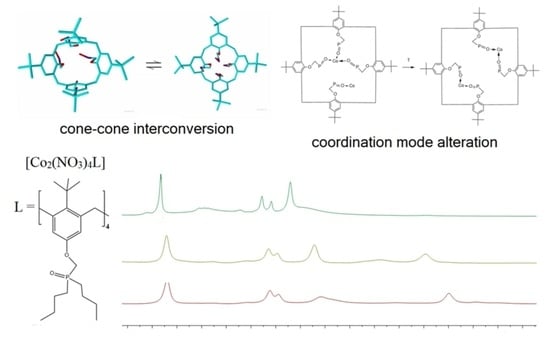
2. Results and Discussion
3. Conclusions
4. Experimental
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Abidi, R.; Baker, M.V.; Harrowfield, J.M.; Ho, D.S.C.; Richmond, W.R.; Skelton, B.W.; White, A.H.; Varnek, A.; Wipff, G. Complexation of the P-t-Butyl-Calix[4]Arene Anion with Alkali Metal Cations in Polar, Non-Aqueous Solvents: Experimental and Theoretical Studies. Inorg. Chim. Acta 1996, 246, 275–286. [Google Scholar] [CrossRef]
- Čajan, M.; Lhoták, P.; Lang, J.; Dvořáková, H.; Stibor, I.; Koča, J. The Conformational Behaviour of ThiaCalix[4]Arenes: The Pinched Cone–Pinched Cone Transition. J. Chem. Soc. Perkin Trans. 2 2002, 2, 1922–1929. [Google Scholar] [CrossRef]
- Bonechi, C.; Donati, A.; Martini, S.; Rossi, C.; Arduini, A.; Pochini, A.; Lonetti, B.; Baglioni, P. Analysis of the P- Tert -ButylCalix[4]Arene Bis-Crown Derivative (Dc3)-Acetonitrile Host−Guest Complexing Behavior by Nuclear Magnetic Resonance (NMR) Spectroscopy and Computational Methods. J. Phys. Chem. B 2004, 108, 7603–7610. [Google Scholar] [CrossRef]
- Gruner, M.; Fischer, C.; Gruber, T.; Weber, E. Influence of Laterally Attached Alkyl Groups on the Conformational Behaviour of a Basic Calix[4]Arene: Combined NMR, Molecular Mechanics and X-Ray Study. Supramol. Chem. 2010, 22, 256–266. [Google Scholar] [CrossRef]
- Andreetti, G.D.; Boehmer, V.; Jordon, J.G.; Tabatabai, M.; Ugozzoli, F.; Vogt, W.; Wolff, A. Dissymmetric Calix[4]Arenes with C4- and C2-Symmetry. Synthesis, x-Ray Structures, Conformational Fixation, and Proton NMR Spectroscopic Studies. J. Org. Chem. 1993, 58, 4023–4032. [Google Scholar] [CrossRef]
- Ikeda, A.; Tsuzuki, H.; Shinkai, S. NMR Spectroscopic and X-Ray Crystallographic Studies of Calix[4]Arene·Ag + Complexes. Influence of Bound Ag + on C 2v –C 2v Interconversion in Cone-Calix[4]Arenes. J. Chem. Soc. Perkin Trans. 2 1994, 2073–2080. [Google Scholar] [CrossRef]
- Horvat, G.; Frkanec, L.; Cindro, N.; Tomišić, V. A Comprehensive Study of the Complexation of Alkali Metal Cations by Lower Rim Calix[4]Arene Amide Derivatives. Phys. Chem. Chem. Phys. 2017, 19, 24316–24329. [Google Scholar] [CrossRef]
- Maji, P.; Krishnamurthy, S.S.; Nethaji, M. NMR and X-Ray Crystallographic Studies of Unsymmetrical 25,26;27,28-Dibridged Para-Tert-Butyl Calix[4]Arene Bisphosphites with a Large “through-Space” P–P Coupling. New J. Chem. 2010, 34, 1478. [Google Scholar] [CrossRef]
- Sarkar, A.; Nethaji, M.; Krishnamurthy, S.S. Phosphite Ligands Derived from Distally and Proximally Substituted Dipropyloxy Calix[4]Arenes and Their Palladium Complexes: Solution Dynamics, Solid-State Structures and Catalysis. J. Organomet. Chem. 2008, 693, 2097–2110. [Google Scholar] [CrossRef]
- Petit, S.; Pilet, G.; Luneau, D.; Chibotaru, L.F.; Ungur, L. A Dinuclear Cobalt(Ii) Complex of Calix[8]Arenes Exibiting Strong Magnetic Anisotropy. Dalt. Trans. 2007, 40, 4582–4588. [Google Scholar] [CrossRef] [Green Version]
- Matt, D.; Steyer, S.; Allouche, L.; Louati, A.; Hamlil, A.; Strehler, C.; Neuburger, M. Conformational Instability of a Proximally-Difunctionalized Calix[4]-Diquinone. J. Mol. Struct. 2005, 740, 53–60. [Google Scholar] [CrossRef]
- Thondorf, I.; Brenn, J. Conformations of Calix[5]Arenes—A Molecular Mechanics Study. J. Chem. Soc. Perkin Trans. 2 1997, 2293–2300. [Google Scholar] [CrossRef]
- Israëli, Y.; Facey, G.A.; Detellier, C. Reorientational Dynamics Ofp-SulfonatoCalix[4]Arene and of Its La(III) Complex in Water. Magn. Reson. Chem. 2004, 42, 573–576. [Google Scholar] [CrossRef]
- Lambert, B.; Jacques, V.; Shivanyuk, A.; Matthews, S.E.; Tunayar, A.; Baaden, M.; Wipff, G.; Böhmer, V.; Desreux, J.F. Calix[4]Arenes as Selective Extracting Agents. An NMR Dynamic and Conformational Investigation of the Lanthanide(III) and Thorium(IV) Complexes. Inorg. Chem. 2000, 39, 2033–2041. [Google Scholar] [CrossRef]
- Fonseca, J.D.; Marcos, P.M.; Ascenso, J.R.; Michel, S.; Hubscher-Bruder, V.; Schurhammer, R. Extraction and Complexation of Lanthanide Ions by DihomooxaCalix[4]Arene and Calix[4]Arene Tetraketone Derivatives: An Experimental and Molecular Dynamics Investigation. Comptes Rendus Chim. 2019, 22, 639–647. [Google Scholar] [CrossRef]
- Kostin, G.A.; US, T.V.; Korda, T.M.; Torgov, V.G.; Kuratieva, N.V.; Miroshnichenko, S.I.; Kalchenko, V.I. Complexation and Extraction of Non-Ferrous Metals by Calix[n]Arene Phosphine Oxides. J. Incl. Phenom. Macrocycl. Chem. 2010, 68, 131–137. [Google Scholar] [CrossRef]
- Sandstrom, J. Dynamic NMR Spectroscopy; Academic Press: London, UK, 1982. [Google Scholar]
- Babailov, S.P.; Mainichev, D.A.; Nikulina, L.D.; Petrova, S.S. Intramolecular Dynamics and Molecular Structure of Europium(III) Chelate Complexes with Crown Ethers as Studied by NMR Spectroscopy. J. Incl. Phenom. Macrocycl. Chem. 2005, 51, 73–78. [Google Scholar] [CrossRef]
- Babailov, S.P.; Stabnikov, P.A.; Korolkov, I.V.; Pervukhina, N.V.; Koshcheeva, O.S.; Chuikov, I.P. Structure and Paramagnetic Properties of Tris -Pivaloyltrifluoracetonate Thulium (III) Complexes with 18-Crown-6 by X-Ray Analysis and NMR. Polyhedron 2016, 105, 178–185. [Google Scholar] [CrossRef]
- Babailov, S.P. Lanthanide Paramagnetic Probes for NMR Spectroscopic Studies of Molecular Conformational Dynamics in Solution: Applications to Macrocyclic Molecules. Prog. Nucl. Magn. Reson. Spectrosc. 2008, 52, 1–21. [Google Scholar] [CrossRef]
- Babailov, S.P.; Stabnikov, P.A.; Zapolotsky, E.N.; Kokovkin, V.V. Lanthanides as NMR Probes of Fast Molecular Dynamics at High Magnetic Fields and Temperature Sensors: Conformational Interconversion of Erbium Ethylenediaminetetraacetate Complexes. Inorg. Chem. 2013, 52, 5564–5569. [Google Scholar] [CrossRef]
- Coman, D.; Trubel, H.K.; Hyder, F. Brain Temperature by Biosensor Imaging of Redundant Deviation in Shifts (BIRDS): Comparison between TmDOTP5− and TmDOTMA−. NMR Biomed. 2010, 23, 277–285. [Google Scholar] [CrossRef] [Green Version]
- Tsitovich, P.B.; Cox, J.M.; Benedict, J.B.; Morrow, J.R. Six-Coordinate Iron(II) and Cobalt(II) ParaSHIFT Agents for Measuring Temperature by Magnetic Resonance Spectroscopy. Inorg. Chem. 2017, 55, 700–716. [Google Scholar] [CrossRef] [Green Version]
- Kostin, G.A.; Borodin, A.O.; Torgov, V.G.; Kuratieva, N.V.; Naumov, D.Y.; Miroshnichenko, S.I.; Kalchenko, V.I. Monomeric and Polymeric Dinuclear Complexes of Co(II) or Ni(II) with Calix[4]Arene-Tetraphosphineoxide. J. Incl. Phenom. Macrocycl. Chem. 2007, 59, 45–52. [Google Scholar] [CrossRef]
- Babailov, S.P.; Zapolotsky, E.N.; Kruppa, A.I.; Stabnikov, P.A.; Godovikov, I.A.; Bocharov, E.V.; Fomin, E.S. Two Types of Conformational Dynamics and Thermo-Sensor Properties of Praseodymium-DOTA by 1H/13C NMR. Inorg. Chim. Acta 2019, 486, 340–344. [Google Scholar] [CrossRef]
- Thondorf, I.; Brenn, J. A Simple Method for the Exploration of the Conformational Space and for the Estimation of Rotational Barriers in Calix[4]Arene Systems. J. Mol. Struct. THEOCHEM 1997, 398–399, 307–314. [Google Scholar] [CrossRef]
- Tlustý, M.; Slavík, P.; Dvořáková, H.; Eigner, V.; Lhoták, P. Synthesis and Study of Calix[4]Arenes Bearing Azo Moieties at the Meta Position. Tetrahedron 2017, 73, 1230–1237. [Google Scholar] [CrossRef]
- Stout, E.W.; Gutowsky, H.S. On the Temperature Dependence of Lanthanide-Induced NMR Shifts. J. Magn. Reson. 1976, 398, 389–398. [Google Scholar] [CrossRef]
- Babailov, S.P.; Peresypkina, E.V.; Journaux, Y.; Vostrikova, K.E. Nickel(II) Complex of a Biradical: Structure, Magnetic Properties, High NMR Temperature Sensitivity and Moderately Fast Molecular Dynamics. Sens. Actuators B Chem. 2017, 239, 405–412. [Google Scholar] [CrossRef]
- Zapolotsky, E.N.; Qu, Y.; Babailov, S.P. Lanthanide Complexes with Polyaminopolycarboxylates as Prospective NMR/MRI Diagnostic Probes: Peculiarities of Molecular Structure, Dynamics and Paramagnetic Properties. J. Incl. Phenom. Macrocycl. Chem. 2021, 102, 1–33. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapolotsky, E.N.; Babailov, S.P.; Kostin, G.A. Paramagnetic Properties and Moderately RapidConformational Dynamics in the Cobalt(II) Calix[4]arene Complex by NMR. Molecules 2022, 27, 4668. https://doi.org/10.3390/molecules27144668
Zapolotsky EN, Babailov SP, Kostin GA. Paramagnetic Properties and Moderately RapidConformational Dynamics in the Cobalt(II) Calix[4]arene Complex by NMR. Molecules. 2022; 27(14):4668. https://doi.org/10.3390/molecules27144668
Chicago/Turabian StyleZapolotsky, Eugeny Nikolaevich, Sergey Pavlovich Babailov, and Gennadiy Alexandrovich Kostin. 2022. "Paramagnetic Properties and Moderately RapidConformational Dynamics in the Cobalt(II) Calix[4]arene Complex by NMR" Molecules 27, no. 14: 4668. https://doi.org/10.3390/molecules27144668
APA StyleZapolotsky, E. N., Babailov, S. P., & Kostin, G. A. (2022). Paramagnetic Properties and Moderately RapidConformational Dynamics in the Cobalt(II) Calix[4]arene Complex by NMR. Molecules, 27(14), 4668. https://doi.org/10.3390/molecules27144668