Identification of Hair Growth Promoting Components in the Kernels of Prunus mira Koehne and Their Mechanism of Action
Abstract
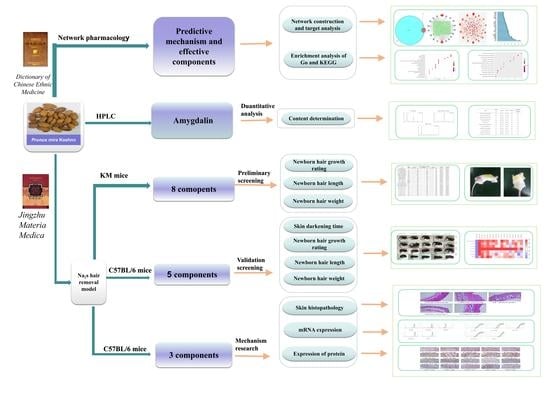
:1. Introduction
2. Material and Methods
2.1. Network Pharmacology Predicts the Mechanism and Active Components of the Kernel of P. mira in the Treatment of Hair Loss
2.2. The Contents of Amygdalin in the Kernel of P. mira Were Determined by HPLC
2.2.1. Materials
2.2.2. Sample Preparation
2.2.3. HPLC Conditions
2.2.4. Preparation and Calibration of Standard Solutions
2.3. Screening of the Effective Components in the Treatment of Two Depilatory Model Animals with Chemical Components in P. mira
2.3.1. Animals and Test Sites
2.3.2. Preparation of Experimental Samples
2.3.3. Preliminary Screening of Eight Components in the Kernel of P. mira in the Treatment of KM Mice after Depilation Induced by Sodium
2.3.4. To Verify the Effect of Five Active Components in the Kernel of P. mira on C57BL/6 Mice after Depilation Induced by Sodium
2.4. Mechanism of the 3 Active Components in the Kernel of P. mira in the Treatment of C57BL/6 Mice Depilation Model Induced by Sodium Sulfide
2.4.1. Grouping and General Indicators
2.4.2. Skin Histological Observation
2.4.3. RT-PCR Detection of mRNA Expression of Four Targets in the Skin Wnt/β-catenin Pathway
2.4.4. Immunohistochemical Method to Detect the Protein Expression of Four Targets in the Skin Wnt/β-catenin Pathway
2.5. Statistical Methods
2.6. Principal Component Analysis Method
3. Results and Discussion
3.1. Network Pharmacology
3.2. HPLC Analysis
3.2.1. Optimization of Chromatographic Separation Conditions
3.2.2. Quantitative Analysis of Amygdalin in the Kernel of P. mira
3.3. Dose-Effect Relationship of Eight Chemical Components in the Kernel of P. mira to Promote Hair Growth
3.4. Re-Evaluating of the Effect of Five Active Components in the Kernel of P. mira in Promoting Hair Growth
3.5. Mechanism of the Main Active Components in the Kernel of P. mira to Promote Hair Growth
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| Akt/Pkb | Recombinant Protein Kinase B |
| β-catenin | Beta chain protein |
| C-myc | proto-oncogene protein |
| EGF | Epidermal Growth Factor |
| EGFR | epidermal growth factor receptor |
| GSK-3β | Glycogen synthase kinase 3 |
| HE | Hematoxylin and eosin |
| HPLC | High Performance Liquid Chromatography |
| LEF 1 | Lymphoid Enhancer-binding Factor 1 |
| PI3K | phosphatidylinositol 3-kinase |
| RT-PCR | Real-time PCR |
| Tcf/Lef | T cell factor/Lymphoid enhancer factor |
| Wnt | Wingless/Integrated |
| IHC | Immunohistochemistry |
| GO | Gene oncology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| HE | hematoxylin-eosin staining |
References
- Ga, W. Jingjing Materia Medica; The Ethnic Publishing House: Beijing, China, 1995. [Google Scholar]
- Zhou, J.; Zhong, Y.; Wang, T.; Yang, S.; Xu, J.; Guo, H. The origin of the type of Prunus mira and peaches in southwest Sichuan. In Proceedings of the A Collection of Papers from the first Youth Symposium of the Chinese Horticultural Society, Hangzhou, China, 4 December 1994; p. 4. [Google Scholar]
- Xu, G.; Xu, L. Variety Sorting and Quality Research of Commonly Used Chinese Medicinal Materials; Fujian Science and Technology Press: Fuzhou, China, 1997. [Google Scholar]
- Dimar, D.Z.P.C. Jingzhu Materia Medica; Shanghai Science and Technology Press: Shanghai, China, 1986. [Google Scholar]
- Jia, M.; Zhang, Y. Dictionary of Chinese Ethnic Medicine; China Medical Science Press: Beijing, China, 2016. [Google Scholar]
- Sichuan Food and Drug Administration. The Standard of Traditional Chinese Medicine in Sichuan 2010; Sichuan Science and Technology Press: Chengdu, China, 2011. [Google Scholar]
- Liang, Y.; Pei, J.; Zhou, M.; Fan, Z.; Liu, W.; Wan, D. Analysis of fat-soluble composition in different varieties of Semen persicae by GC-MS. West China J. Pharm. Sci. 2012, 27, 174–176. [Google Scholar]
- Li, B. A Study on Key Issues in China’s Implementation of the Nagoya Protocol on Access to and Benefit Sharing of Genetic Resources. Ph.D. Thesis, Minzu University of China, Beijing, China, 2020. [Google Scholar]
- Pei, S. Discussion on Ethnic Medicine research and New Drug Development in China (I). J. Yunnan Univ. Tradit. Chin. Med. 2007, 30, 1–4. [Google Scholar]
- Zhong, Z.; Pu, Q. Cool Tibetan wild peach fruit processing technology research. For. Sci. Technol. 2001, 44, 35–36. [Google Scholar] [CrossRef]
- Cai, C.; Zhong, M.; Huang, B.; Wang, B.; Xiao, W.; Qiu, J. Tibetan wild peach resources and their comprehensive processing and utilization. Guangdong Agric. Sci. 1997, 33, 25–26. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, R.; Lu, G. Economic evaluation and protection of Amygdalus mira genetic resource. Acta Ecol. Sin. 2013, 33, 7277–7287. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, X.; Wang, Z.; Zhang, J.; Yue, M.; Liang, Y.; Fan, G.; Yin, H. Analysis of fat-soluble components of nuts oil from Prunus mira in Derong County by GC-MS. Chin. Tradit. Pat. Med. 2018, 40, 142–146. [Google Scholar]
- Liang, Y. Study on Quality Evaluation of the Effective Components in Safflower-Semen Persicae and Their Preparations. Master’s Thesis, Chengdu University of TCM, Chengdu, China, 2011. [Google Scholar]
- Stenn, K. Bioengineering the human cells. Investig. Dermatol. 2007, 127, 2106–2115. [Google Scholar]
- Teng, X. Positional Cloning of a Novel Gene in Uncoverd Mouse and Functional Analysis in Mouse hair Follicle Development. Ph.D. Thesis, Shanghai Jiao Tong University, Shanghai, China, 2008. [Google Scholar]
- Camacho, F.; Montagna, W. Current Concept and Classification. Male Androgenetic Alopecia; Group Aula Mdica SA: Madrid, Spain, 1997; pp. 325–342. [Google Scholar]
- Chen, Q. The Traditional Chinese Medicine Efficacy Research Ideas and Methods; People’s Medical Publishing House: Beijing, China, 2004. [Google Scholar]
- Huo, T.; Fang, Y.; Zhao, L.; Xiong, Z.; Zhang, Y.; Wang, Y.; Feng, C.; Yuan, M.; Wang, S.; Chen, M.; et al. HNMR-based metabonomic study of sub-chronic hepatotoxicity induced by realgar. J. Ethnopharmacol. 2016, 192, 1–9. [Google Scholar] [CrossRef]
- Dang, J.; Yu, L.; Wang, Y. Study on treatment of Pterocarpus indcus Willd extracts on rat androgenetic alopecia. West China J. Pharm. Sci. 2010, 25, 320–322. [Google Scholar]
- Hu, X.; Wang, W.; Zhong, Q.; Yu, B.; Ma, G. Questionnaire analysis of related factors of male pattern alopecia and clinical efficacy of finasteride therapy in 320 patients. J. Xi’an Jiaotong Univ. (Med. Sci.) 2009, 30, 620–623. [Google Scholar]
- Rushton, D.; Norris, M.; Dover, R.; Busuttil, N. Causes of hair loss and the developments in hair rejuvenation. Int. J. Cosmet. Sci. 2002, 24, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Castelli, E.; Fiorella, S.; Caputo, V. Pili annulati coincident with alopecia areata, autoimmune thyroid disease, and primary IgA deficiency: Case report and considerations on the literature. Case Rep. Dermatol. 2012, 4, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Liu, T. The research progress of traditional Chinese medicine in the treatment of seborrheic alopecia. Hunan J. Tradit. Chin. Med. 2014, 30, 186–187. [Google Scholar]
- Rossi, A.; Cantisani, C.; Melis, L.; Iorio, A.; Scali, E.; Calvieri, S. Minoxidil use in dermatology, side effects and recent patents. Recent Pat. Inflamm. Allergy Drug Discov. 2012, 6, 130–136. [Google Scholar] [CrossRef]
- Irwig, M.S.; Kolukula, S. Persistent sexual side effects of finasteride for male pattern hair loss. J. Sex Med. 2011, 8, 1747–1753. [Google Scholar] [CrossRef]
- Zhou, W. Literature Research on Traditional Chinese Medicine in the Treatment of Hair Loss in the Past 50 years. Master’s Thesis, Nanjing University of Chinese Medicine, Nanjing, China, 2016. [Google Scholar]
- Luo, J. Clinical Observation on 18 Cases of Alopecia areata Treated with Plum Blossom Needle Tap and Vitamin E External Rubbing. In Proceedings of the 2013 Annual Conference of Gansu Society of Traditional Chinese Medicine, Qingyang, China, 26 July 2013; p. 1. [Google Scholar]
- Zhang, N.; Yang, Y.; Duan, Q. Research progress of local and external treatment of androgen-induced alopecia with traditional Chinese and western medicine. J. Ext. Ther. Tradit. Chin. Med. 2018, 27, 52–54. [Google Scholar]
- Bunagan, M.J.K.S. Medical Treatment of Hair Loss. In Hair Restoration Surgery in Asians; Springer: Tokyo, Japan; Berlin/Heidelberg, Germany; New York, NY, USA, 2010. [Google Scholar]
- Tao, J.; Wang, S.; Chen, Y.; Zheng, H.; Jiang, M.; Yuan, B. Review on the research in network pharmacology of traditional Chinese medicine compound. China J. Tradit. Chin. Med. Pharm. 2019, 34, 3903–3907. [Google Scholar]
- Zhou, Y. Research on the Optimal Components and Integrated Regulation Mechanism of Fufangshuangdanfang (SDF) in Improving Diabetic Kidney Disease Based on Pathology Network. Master’s Thesis, Shanxi University of Chinese Medicine, Taiyuan, China, 2018. [Google Scholar]
- Xin, W.; Bai, M.; Miao, M. Chinese Medicine Research and the Secondary Development Model of Traditional Chinese Medicine Based on Network Pharmacology. Acta Chin. Med. 2016, 31, 92–95. [Google Scholar]
- Song, K.; Bi, T.; Zhan, X.; Li, Z.; Wang, J.; He, W.; Tong, Y.; Li, Y. Strategy of Discovering Active Ingredient in Traditional Chinese Medicine under Guidance of Network Pharmacology. Mod. Tradit. Chin. Med. Mater. Med. World Sci. Technol. 2014, 16, 27–31. [Google Scholar]
- Wu, G.; Zheng, L.; Ma, Q.; Han, Z.; Zhan, R.; Chen, W.; NIU, M. Targets prediction and mechanism of Zanthoxylum nitidum in intervention of inflammation based on network pharmacology. Chin. Tradit. Herb. Drugs 2019, 50, 659–668. [Google Scholar]
- Tao, W.; Xu, X.; Wang, X.; Li, B.; Wang, Y.; Li, Y.; Yang, L. Network pharmacology-based prediction of the active ingredients and potential targets of Chinese herbal Radix Curcumae formula for application to cardiovascular disease. J. Ethnopharmacol. 2013, 145, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Huang, S.; Peng, D.; Han, L.; Wang, X.; Wang, Y.; Pan, L. Application of network pharmacology in the study of traditional Chinese medicine formula. Chin. Pharmacol. Bull. 2020, 36, 303–308. [Google Scholar]
- Yuan, C.; Liu, B.; Huang, J.; Yan, Z.; Chen, R.; Huo, L. Application of Network Pharmacology on Screening and Mechanism of Pharmacodynamic Substances of Traditional Chinese Medicine. Guangzhou Chem. Ind. 2019, 47, 20–22. [Google Scholar]
- Zhou, Y.; Tang, G.; Li, X.; Sun, W.; Liang, Y.; Gan, D.; Liu, G.; Song, W.; Wang, Z. Study on the chemical constituents of nut oil from Prunus mira Koehne and the mechanism of promoting hair growth. J. Ethnopharmacol. 2020, 258, 112831. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, X.; Wang, Z.; Zhang, J.; Gan, D.; Liang, Y.; Fan, G.; Yin, H. Acute toxicity test of light walnut kernel oil on experimental mice and rabbits. China Med. Bull. 2017, 14, 18–24. [Google Scholar]
- Luo, S.; Deng, y.; Zhu, Y. Determination of Oleic Acid and Linoleic Acid in the Oil of Fructus Bruceae by Pre-column Derivatization HPLC. Tradit. Chin. Drug Res. Clin. Pharmacol. 2011, 22, 328–330. [Google Scholar] [CrossRef]
- Kang, J.-I.; Kim, E.-J.; Kim, M.-K.; Jeon, Y.-J.; Kang, S.-M.; Koh, Y.-S.; Yoo, E.-S.; Kang, H.-K. The promoting effect of Ishige sinicola on hair growth. Mar. Drugs 2013, 11, 1783–1799. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qi, H.; Li, J.; Yi, Y.; Chen, D.; Hu, X.; Wang, M.; Sun, X.; Wei, X. Experimental study on Dendrobium candidum polysaccharides on promotion of hair growth. China J. Chin. Mater. Med. 2014, 39, 291–295. [Google Scholar] [CrossRef]
- Tie, R.; Liu, L.; Li, X.; Yu, J.; Qu, P.; Zhou, X.; Zhang, X. The study of sodium sulfide depilatory optimum unhairing concentration. Shanxi Med. J. 2009, 38, 1283–1284+1303. [Google Scholar] [CrossRef]
- Li, Y. Experimental Methodology of Traditional Chinese Medicine Pharmacology Version 2; Shanghai Science and Technology Press: Shanghai, China, 2006. [Google Scholar]
- Zhang, L. Pharmacodynamic Evaluation and Mechanism Research of Chinese Medicine Hair Restore on the Treatment of Seborrheic alopecia. Master’s Thesis, Guangzhou University of Chinese Medicine, Guangzhou, China, 2013. [Google Scholar]
- Xia, T.; Li, W.; Xia, X.; He, W.; Zhu, X. The Research on the Promoting Effect of Ginkgo Pilatory Mixture on Hair Growth in Mice. Guangxi J. Tradit. Chin. Med. 2013, 36, 69–72. [Google Scholar] [CrossRef]
- 48. Yingying. Study on Pharmacodynamics of Dong Gong Hair Growth and Blacking Lotion. Master’s Thesis, Jilin University, Changchun, China, 2009.
- Yang, S.; Ma, S.; Zhong, Z.; Qin, J.; Li, Z.; Wu, L. Establishment of Hair Cycle Animal Model with C57BL6 Mice. Chin. J. Dermatol. 1999, 32, 34–35. [Google Scholar]
- Polakowska, R.R.; Piacentini, M.; Bartlett, R.; Goldsmith, L.A.; Haake, A.R. Apoptosis in human skin development: Morphogenesis, periderm, and stem cells. Dev. Dyn. 1994, 199, 176–188. [Google Scholar] [CrossRef]
- Xing, F.; Yi, W.-J.; Miao, F.; Su, M.-Y.; Lei, T.-C. Baicalin increases hair follicle development by increasing canonical Wnt/β-catenin signaling and activating dermal papillar cells in mice. Int. J. Mol. Med. 2018, 41, 2079–2085. [Google Scholar] [CrossRef]
- Guo, Q.; Xing, X.; Wu, H. Regulation of Wnt Signaling Pathway by Protein Modification. Chin. J. Biochem. Mol. Biol. 2009, 25, 110–115. [Google Scholar]
- Gao, N.; Cao, Y.; Liu, T.; Pang, J.; Zhan, H.; Shi, Y. Wnt signaling pathways and osteoarthritis. China J. Orthopaed. Traumatol. 2010, 23, 320–323. [Google Scholar]
- Yue, Q.; Liang, M. Regulative effect of the canonical Wnt pathway on osteogenesis. Int. J. Biomed. Eng. 2014, 37, 242–246. [Google Scholar]
- Wang, M. The effect of Wnt Signaling Pathway and TCF7L2 on Type 2 Diabetes. Ph.D. Thesis, Fu Dan University, Shanghai, China, 2010. [Google Scholar]
- Li, Y. The Mechanisms of Hair Follicle Cycle Regulated by Wnt10b. Ph.D. Thesis, Third Military Medical University, Chongqing, China, 2011. [Google Scholar]
- Ni, C.; Yu, M.; Zhang, Z. The role of EGFR and PI3K/AKT signal pathway in non-small cell lung cancer. Acta Univ. Med. Anhui 2011, 46, 1264–1266. [Google Scholar]
- Pan, R.; Jin, Y.; Zhu, W. Advances have been made in the study of apoptosis mechanisms mediated by PI3K/Akt signal path paths. Zhejiang J. Integr. Trad. Chin. West. Med. 2013, 23, 70–72. [Google Scholar]
- Chen, J.; Jiang, C.; Fu, L.; Zhu, C.-L.; Xiang, Y.-Q.; Jiang, L.-X.; Chen, Q.; Liu, W.M.; Chen, J.-N.; Zhang, L.-Y.; et al. suppresses tumor growth and metastasis in nasopharyngeal carcinoma by repressing PI3K/AKT signaling pathway via interaction with Integrin β1 and Merlin. Int. J. Biol. Sci. 2019, 15, 1802–1815. [Google Scholar] [CrossRef]
- Chen, H.-W.; Lin, A.-H.; Chu, H.-C.; Li, C.-C.; Tsai, C.-W.; Chao, C.-Y.; Wang, C.-J.; Lii, C.-K.; Liu, K.-L. Inhibition of TNF-α-Induced Inflammation by andrographolide via down-regulation of the PI3K/Akt signaling pathway. J. Nat. Prod. 2011, 74, 2408–2413. [Google Scholar] [CrossRef]
- Watabe, H.; Furuhama, T.; Tani-Ishii, N.; Mikuni-Takagaki, Y. Mechanotransduction activates α₅β₁ integrin and PI3K/Akt signaling pathways in mandibular osteoblasts. Exp. Cell Res. 2011, 317, 2642–2649. [Google Scholar] [CrossRef]
- Zhang, W.; Miao, L. PI3K/Akt signaling path and its progress in neuropathy. J. Apoplexy Nerv. Dis. 2007, 24, 755–757. [Google Scholar]
- Zhang, H.; Su, Y.; Wang, J.; Gao, Y.; Yang, F.; Li, G.; Shi, Q. Ginsenoside Rb1 promotes the growth of mink hair follicle via PI3K/AKT/GSK-3β signaling pathway. Life Sci. 2019, 229, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Shigematsu, H.; Nakajima, T.; Kubo, R.; Motohashi, S.; Sekine, Y.; Shibuya, K.; Iizasa, T.; Hiroshima, K.; Nakatani, Y.; et al. Synchronous alterations of Wnt and epidermal growth factor receptor signaling pathways through aberrant methylation and mutation in non small cell lung cancer. Clin. Cancer Res. 2007, 13, 6087–6092. [Google Scholar] [CrossRef] [PubMed]
- Civenni, G.; Holbro, T.; Hynes, N.E. Wnt1 and Wnt5a induce cyclin D1 expression through ErbB1 transactivation in HC11 mammary epithelial cells. EMBO Rep. 2003, 4, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Weber, U.; Pataki, C.; Mihaly, J.; Mlodzik, M. Combinatorial signaling by the Frizzled/PCP and Egfr pathways during planar cell polarity establishment in the Drosophila eye. Dev. Biol. 2008, 316, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, J.; Shi, C.; Wang, Y.; Yang, J.; Yang, T. Regulatory effect of β-catenin on proliferation of hair follicle stem cells involves PI3K/Akt pathway. J. Appl. Biomed. 2013, 11, 131–141. [Google Scholar] [CrossRef]
- Westerberg, R.; Tvrdik, P.; Undén, A.-B.; Månsson, J.-E.; Norlén, L.; Jakobsson, A.; Holleran, W.H.; Elias, P.M.; Asadi, A.; Flodby, P.; et al. Role for ELOVL3 and fatty acid chain length in development of hair and skin function. J. Biol. Chem. 2004, 279, 5621–5629. [Google Scholar] [CrossRef]
- Truong, V.-L.; Bak, M.J.; Lee, C.; Jun, M.; Jeong, W.-S. Hair Regenerative Mechanisms of Red Ginseng Oil and Its Major Components in the Testosterone-Induced Delay of Anagen Entry in C57BL/6 Mice. Molecules 2017, 22, 1505. [Google Scholar] [CrossRef]
- Upadhyay, K.; Gupta, N.K.; Dixit, V.K. Development and characterization of phyto-vesicles of β-sitosterol for the treatment of androgenetic alopecia. Arch. Dermatol. Res. 2012, 304, 511–519. [Google Scholar] [CrossRef]
- Tong, T.; Kim, N.; Park, T. Topical Application of Oleuropein Induces Anagen Hair Growth in Telogen Mouse Skin. PLoS ONE 2015, 10, e0129578. [Google Scholar] [CrossRef] [PubMed]
- Vingler, P.; Gautier, B.; Dalko, M.; Rozot, R.; Gaillard, O.; Michelet, J.F.; Bernard, B.A. 6-O glucose linoleate supports in vitro human hair growth and lipid synthesis. Int. Cosmet. Sci. 2007, 29, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Z.; Feng, Y.; Hao, H. The efficacy of vitamin E injection local injection to treat baldness was observed. Chin. J. Clin. Ration. Drug Use 2014, 7, 181. [Google Scholar]
- Clevers, H.; Nusse, R. Wnt/β-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Lei, M. The mechanism of Volatile oil from Platyclsdus orientalis Leaves to Induce Hair Growth in C57BL/6 Mice. Master’s Thesis, Northwest University, Kirkland, WA, USA, 2018. [Google Scholar]
- Gan, X.-q.; Wang, J.-y.; Xi, Y.; Wu, Z.-l.; Li, Y.-p.; Li, L. Nuclear Dvl, c-Jun, beta-catenin, and TCF form a complex leading to stabilization of beta-catenin-TCF interaction. J. Cell Biol 2008, 180, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-h.; Xiang, L.-J.; Shi, H.-X.; Zhang, J.; Jiang, L.-p.; Cai, P.-t.; Lin, Z.-L.; Lin, B.-B.; Huang, Y.; Zhang, H.-L.; et al. Fibroblast growth factors stimulate hair growth through β-catenin and Shh expression in C57BL/6 mice. Biomed. Res. Int. 2015, 2015, 730139. [Google Scholar] [CrossRef]
- Xu, X.; Lyle, S.; Liu, Y.; Solky, B.; Cotsarelis, G. Differential expression of cyclin D1 in the human hair follicle. Am. J. Pathol. 2003, 163, 969–978. [Google Scholar] [CrossRef]
- Bai, T.; Liu, F.; Zou, F.; Zhao, G.; Jiang, Y.; Liu, L.; Shi, J.; Hao, D.; Zhang, Q.; Zheng, T.; et al. Epidermal Growth Factor Induces Proliferation of Hair Follicle-Derived Mesenchymal Stem Cells through Epidermal Growth Factor Receptor-Mediated Activation of ERK and AKT Signaling Pathways Associated with Upregulation of Cyclin D1 and Downregulation of p16. Stem Cells Dev. 2017, 26, 113–122. [Google Scholar] [CrossRef]
- Sun, W.; Gan, D.; Wang, Z.; Zhang, J.; Fan, G.; Yin, H. Study on the data mining of animal model of alopecia and medical treatment. Pharm. Clin. Chin. Mater. Med. 2017, 8, 57–61. [Google Scholar]
- Yue, X.; Lan, F.; Yang, W.; Yang, Y.; Han, L.; Zhang, A.; Liu, J.; Zeng, H.; Jiang, T.; Pu, P.; et al. Interruption of β-catenin suppresses the EGFR pathway by blocking multiple oncogenic targets in human glioma cells. Brain Res. 2010, 1366, 27–37. [Google Scholar] [CrossRef]
- Huang, L.; Li, P.; Xia, X.; He, w.; Zhu, X. Effect of Ginkgo Mixture on Hair Growth Promoting in C57BL/6 Mice. J. Liaoning Univ. Tradit. Chin. Med. 2013, 15, 65–67. [Google Scholar]
- Wang, H. Effect of 8DSS on Hair Growth and Its Possible Mechanisms. Ph.D. Thesis, Third Military Medical University, Chongqing, China, 2015. [Google Scholar]
- Commission, N.P. Pharmacopoeia of the People’s Republic of China: One in 2015; China Medical Science Press: Beijing, China, 2015. [Google Scholar]
- Yang, Y.; Di, S.; Zhang, H.; Zhao, L.; Tong, X. Study on clinical application and dosage of peach kernel. Jilin J. Chin. Med. 2018, 38, 1441–1446. [Google Scholar] [CrossRef]
- Du, H.; Xu, T.; Li, Q.; Wang, Z.; Zhang, J.; Fan, G. Prunus mira Kernels compared with peach kernel HPLC fingerprint. Chin. Tradit. Pat. Med. 2019, 41, 2796–2799. [Google Scholar]
- Li, L.; Liu, Y.; Yan, Y.; Tang, Z.; Duan, J.; Song, Z.; Yang, S.; Liu, F.; Chen, Y.; Xu, G. Research on grade prediction of Persicae semen based on binary Logistic regression analysis. Chin Tradit. Herb. Drugs 2019, 50, 4691–4696. [Google Scholar]

















| Tools | Function | Website |
|---|---|---|
| Pubchem | Compound structure search | https://pubchem.ncbi.nlm.nih.gov (accessed on 8 May 2020) |
| Pharmmapper | Compound target prediction | http://www.lilab-ecust.cn/Pharmmapper (accessed on 8 May 2020) |
| UniProt | Protein name correction | https://www.Uniprot.org (accessed on 10 May 2020) |
| Genecards | Disease target prediction | http://www.genecards.org (accessed on 13 May 2020) |
| String | Construct protein interaction map (PPI) | https://String—db.org (accessed on 23 May 2020) |
| Venny 2.1 | Drawing | https://bioinfogp.cnb.csic.es/tools/Venny/ (accessed on 17 May 2020) |
| Metascape | Analyze the database | https://metascape.org/gp/index.html (accessed on 25 May 2020) |
| Cytospase3.7.1 | Drawing software | —— |
| Group | Dose (mg/cm2/d) | Dose Volume (mL/cm2/d) | Drug Concentration (mg/mL) | Group | Dose (mg/cm2/d) | Dose Volume (mL/cm2/d) | Drug Concentration (mg/mL) |
|---|---|---|---|---|---|---|---|
| Blank group | — | 0.025 | — | Model control group | — | 0.025 | — |
| Minoxidi control group | 0.500 | 0.025 | 20.00 | — | — | — | — |
| β-sitosterol group 1 | 0.123 | 0.025 | 4.90 | linoleic acid group 1 | 0.313 | 0.025 | 12.50 |
| β-sitosterol group 2 | 0.061 | 0.025 | 2.45 | linoleic acid group 2 | 0.156 | 0.025 | 6.25 |
| β-sitosterol group 3 | 0.031 | 0.025 | 1.23 | linoleic acid group 3 | 0.078 | 0.025 | 3.13 |
| β-sitosterol group 4 | 0.016 | 0.025 | 0.62 | linoleic acid group 4 | 0.039 | 0.025 | 1.57 |
| β-sitosterol group 5 | 0.008 | 0.025 | 0.31 | linoleic acid group 5 | 0.020 | 0.025 | 0.79 |
| vitamin E group 1 | 6.250 | 0.025 | 250.00 | oleic acid group 1 | 0.00055 | 0.025 | 0.02200 |
| vitamin E group 2 | 3.125 | 0.025 | 125.00 | oleic acid group 2 | 0.00028 | 0.025 | 0.01100 |
| vitamin E group 3 | 1.563 | 0.025 | 62.50 | oleic acid group 3 | 0.00015 | 0.025 | 0.00600 |
| vitamin E group 4 | 0.781 | 0.025 | 31.25 | oleic acid group 4 | 0.00008 | 0.025 | 0.00300 |
| vitamin E group 5 | 0.391 | 0.025 | 15.63 | oleic acid group 5 | 0.00004 | 0.025 | 0.00150 |
| Trans squalene group 1 | 0.123 | 0.025 | 4.90 | Campesterol group 1 | 0.060 | 0.025 | 2.39 |
| Trans squalene group 2 | 0.061 | 0.025 | 2.45 | Campesterol group 2 | 0.030 | 0.025 | 1.20 |
| Trans squalene group 3 | 0.031 | 0.025 | 1.23 | Campesterol group 3 | 0.015 | 0.025 | 0.60 |
| Trans squalene group 4 | 0.016 | 0.025 | 0.62 | Campesterol group 4 | 0.008 | 0.025 | 0.30 |
| Trans squalene group 5 | 0.008 | 0.025 | 0.31 | Campesterol group 5 | 0.004 | 0.025 | 0.15 |
| Fucosterol group 1 | 0.123 | 0.025 | 4.90 | Amygdalin group 1 | 0.123 | 0.025 | 4.90 |
| Fucosterol group 2 | 0.061 | 0.025 | 2.45 | Amygdalin group 2 | 0.061 | 0.025 | 2.45 |
| Fucosterol group 3 | 0.031 | 0.025 | 1.23 | Amygdalin group 3 | 0.031 | 0.025 | 1.23 |
| Fucosterol group 4 | 0.016 | 0.025 | 0.62 | Amygdalin group 4 | 0.016 | 0.025 | 0.62 |
| Fucosterol group 5 | 0.008 | 0.025 | 0.31 | Amygdalin group 5 | 0.008 | 0.025 | 0.31 |
| Rating | Standard (KM Mice) | Standard (C57BL/6 Mice) |
|---|---|---|
| I | Hairless growth | The skin of the administration area is pink |
| II | Shallow hair overgrown depilated area | The skin in the depilatory area is gray |
| III | The length and density of new hair is about one-half of the unhaired area | The skin in the epilation area is black |
| IV | No difference between newborn hair growth and unhaired areas | Hair grows in the depilatory area |
| Reagent | Volume (μL) |
|---|---|
| 5 × gDNA Eraser Buffer | 2 |
| gDNA Eraser | 1 |
| Total RNA | 2 |
| RNase Free dH2O | 5 |
| Total | 10 |
| Reagent | Volume (μL) |
|---|---|
| 5 × PrimeScript Buffer 2 | 4 |
| PrimeScript RT Enzyme Mix I | 1 |
| RT Primer Mix | 1 |
| RNA | 10 |
| RNase Free dH2O | 4 |
| Total | 20 |
| Primer Name | Upstream | Downstream | Citation Length, bp |
|---|---|---|---|
| β-actin | gaagatcaagatcattgctcc | tactcctgcttgctgatcca | 111 |
| Cyclin D 1 | ccagaggcggatgagaacaagcagac | tgtgcggtagcaggagaggaagttgt | 183 |
| GSK3β | acagtggtgtggatcagttggtggaa | ccagaggcggatgagaacaagcagac | 153 |
| LEF 1 | caacgggcatgaggtggtcagacaag | agtgctcgtcgctgtaggtgatgagg | 293 |
| β-actin | gaagatcaagatcattgctcc | tactcctgcttgctgatcca | 296 |
| Reagent | Volume (μL) |
|---|---|
| 2 × Real PCR EasyTM Mix-SYBR | 10.0 |
| Forward Primer (10 μM) | 0.8 |
| Reverse Primer (10 μM) | 0.8 |
| Template (DNA) | 2.0 |
| ddH2O | 6.4 |
| Total | 20.0 |
| Reaction Temperature | Time | Remarks |
|---|---|---|
| 95 °C | 30 s | Predenaturation |
| 95 °C | 5 s | transsexual |
| 55 °C | 30 s | annealing |
| 72 °C | 30 s | Fully extend to collect fluorescence |
| Compound | CID | Compound | CID |
|---|---|---|---|
| amygdalin | 656516 | arachidic acid | 10467 |
| beta-sitosterol | 222284 | campesterol | 173183 |
| cyclododecene | 637538 | fucosterol | 5281328 |
| glyceryl monooleatemonooleate | 5283468 | methyl linoleate | 5284421 |
| methyl oleate | 5364509 | myristic acid | 11005 |
| lauric acid | 3893 | linoleic acid | 5280450 |
| oleic acid | 445639 | squalene | 638072 |
| stearic acid | 5281 | trans-squalene | 638072 |
| vitamin E | 14985 |
| Gene Name | Gene Name | Gene Name | Gene Name | Gene Name | Gene Name |
|---|---|---|---|---|---|
| ABO | ACADVL | ACHE | ALAD | ALOX12 | APIP |
| AR | ARHGAP11 | ARHGEF12 | ARL2 | AZGP1 | B2M |
| CALmL3 | CAMK4 | CAPN2 | CAPN9 | CCND1 | CCNE1 |
| CD1B | CD2 | CD8A | CDCA8 | CDKN1B | CEL |
| CES1 | CETN2 | CMAS | CREBBP | CUL1 | CYB5A |
| CYB5B | CYB5R3 | CYCS | CYP2E1 | DDC | DGCR8 |
| DHPS | DLG1 | DRAP1 | E2F1 | ECHS1 | EDC3 |
| EEF1A1 | EGF | EIF2AK2 | EIF4A1 | EIF4E | EP300 |
| ESR2 | ETFB | EZR | F2 | FLOT2 | FUT8 |
| GAD1 | GC | GCDH | GLB1 | GLYCTK | GOT2 |
| GSS | GSTA1 | GYG1 | HBA2 | HBB | HEXA |
| HMOX1 | HSD17B11 | HSD17B4 | HSP90AB1 | KCNIP3 | LAP3 |
| LDHC | LIG1 | LIG4 | LTF | MAGEA4 | MAGI2 |
| MAN2A1 | MAN2B1 | MB | MDM2 | MEX3D | MMP2 |
| MOG | MRI1 | MTAP | MUC1 | NGLY1 | NPL |
| NR1I3 | NUCB1 | OGG1 | P4HB | PAPOLA | PEBP1 |
| PFN2 | PHGDH | PMM1 | POLRMT | POT1 | PPP1R8 |
| PPP2R1A | PRPS1 | PTGS1 | PTPRJ | PTPRN | RAB5C |
| RAB7A | RAC1 | RANBP2 | RARG | RBP4 | RGS18 |
| RGS6 | RHO | RPS3 | RRM1 | RUNX1T1 | RXRB |
| RXRG | S100A11 | S100A6 | S100A8 | S100B | SCO2 |
| SDHA | SF1 | SIN3A | SMS | SOD2 | SRP54 |
| SUB1 | SUOX | TCEA3 | THBS1 | THRB | TJP1 |
| TOX | TRPV6 | TUFM | TXNRD1 | UNG | USF1 |
| USH1C | USP14 | USP19 | USP8 | VCP | — |
| Chemical Component | Linear Regression Equation | Correlation Coefficient (R) | Linear Range (µg/mL) | Precision | Repeatability | Stability | Average Value of Sample Recovery | Sample Recovery Rate |
|---|---|---|---|---|---|---|---|---|
| Amygdalin | y = 10749x + 1018982 | 0.9993 | 80.00–1280.00 | 2.60% | 2.40% | 2.8% | 95.04% | 1.60% |
| Origin | Altitude (m) | Longitude | Latitude | Oil Yield (%) | Content (mg/g) |
|---|---|---|---|---|---|
| Amygdalin | |||||
| Xulong Township, Derong County | 2935 | 99°13′7005″ | 28°74′1808″ | 38.08 | 14.8 ± 0.2 |
| Zhongza Town, Batang County | 2929 | 99°19′1089″ | 29°21′2186″ | 37.89 | 11.0 ± 1.8 |
| Bajiaolou Township, Yajiang County | 2719 | 101°06′1872″ | 30°06′0709″ | 37.94 | 15.1 ± 0.3 |
| Chitu Township, Daocheng County | 3164 | 100°16′1899″ | 28°37′3726″ | 38.13 | 16.2 ± 0.3 |
| Malkang Forestry Bureau | 2630 | 102°13′4203″ | 31°55′2159″ | 38.11 | 13.9 ± 0.7 |
| Xinlong County | 3066 | 100°31′1368″ | 30°93′9169″ | 38.04 | 25.9 ± 0.7 |
| Zhengdou Township, Xiangcheng County | 2750 | 99°31′2148″ | 29°05′4042″ | 37.53 | 11.1 ± 2.7 |
| Wachang Town, Muli County | 2577 | 100°50′2772″ | 28°09′4138″ | 38.23 | 15.1 ± 4.8 |
| Pusharong Township, Kangding City | 2922 | 101°19′1446″ | 29°32′1447″ | 38.05 | 19.6 ± 0.1 |
| Jiaer Town, Jiulong County | 2823 | 101°30′3892″ | 28°59′1512″ | 38.04 | 21.2 ± 0.3 |
| Nixi Township, Shangri-La City * | 3135 | 99°50′6456″ | 28°04′7398″ | 37.87 | 12.1 ± 1.8 |
| Benzilan Town, Deqin County * | 2220 | 99°16′4288″ | 28°14′2346″ | 37.94 | 13.5 ± 2.7 |
| Group | Dose (mg/cm2/d) | Newborn Hair Growth Rating | Newborn Hair Length (mm) | Newborn Hair Weight (mg) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 2 | Day 4 | Day 6 | ||||||||||||||||
| I | II | III | IV | p | I | II | III | IV | p | I | II | III | IV | p | ||||
| Blank group | — | 5 | 5 | 0 | 0 | 0.660 | 0 | 3 | 7 | 0 | 0.024 * | 0 | 0 | 3 | 7 | 0.129 * | 6.72 ± 0.53 ** | 2.82 ± 1.26 ** |
| Model control group | — | 7 | 3 | 0 | 0 | — | 0 | 8 | 2 | 0 | — | 0 | 2 | 4 | 4 | — | 3.73 ± 1.7 | 1.33 ± 0.59 |
| Minoxidi control group | 0.500 | 7 | 3 | 0 | 0 | 1.000 | 0 | 2 | 5 | 3 | 0.003 ** | 0 | 0 | 3 | 7 | 0.129 | 4.63 ± 0.85 | 2.03 ± 0.51 * |
| β-sitosterol group 1 | 0.123 | 8 | 2 | 0 | 0 | 0.016 * | 3 | 4 | 3 | 0 | 0.113 | 1 | 3 | 5 | 1 | 0.129 | 3.64 ± 1.70 | 1.86 ± 0.74 |
| β-sitosterol group 2 | 0.061 | 9 | 1 | 0 | 0 | 0.001 * | 0 | 4 | 6 | 0 | 0.044 * | 0 | 4 | 4 | 2 | 0.227 | 3.91 ± 1.95 | 2.38 ± 1.27 * |
| β-sitosterol group 3 | 0.031 | 8 | 2 | 0 | 0 | 0.016 * | 0 | 7 | 3 | 0 | 0.628 * | 0 | 2 | 6 | 2 | 0.262 | 3.51 ± 0.55 | 1.95 ± 0.98 |
| β-sitosterol group 4 | 0.016 | 7 | 3 | 0 | 0 | 1.000 | 0 | 7 | 3 | 0 | 0.628 * | 0 | 3 | 6 | 1 | 0.227 | 3.23 ± 1.42 | 1.49 ± 0.75 |
| β-sitosterol group 5 | 0.008 | 7 | 3 | 0 | 0 | 1.000 | 1 | 2 | 6 | 1 | 0.063 * | 1 | 1 | 3 | 5 | 0.815 | 4.02 ± 1.83 | 2.20 ± 1.36 |
| linoleic acid group 1 | 0.313 | 2 | 8 | 0 | 0 | 0.064 | 1 | 6 | 3 | 0 | 0.928 | 2 | 3 | 3 | 2 | 0.137 | 3.35 ± 2.69 | 1.64 ± 1.42 |
| linoleic acid group 2 | 0.156 | 0 | 7 | 3 | 0 | 0.021 * | 0 | 2 | 8 | 0 | 0.005 ** | 0 | 2 | 2 | 6 | 0.525 | 5.33 ± 1.69 | 1.82 ± 1.42 |
| linoleic acid group 3 | 0.078 | 1 | 7 | 2 | 0 | 0.003 ** | 1 | 3 | 6 | 0 | 0.177 | 1 | 1 | 4 | 4 | 0.380 | 4.28 ± 2.11 | 1.64 ± 1.11 |
| linoleic acid group 4 | 0.039 | 3 | 7 | 0 | 0 | 0.081 | 1 | 4 | 5 | 0 | 0.350 | 1 | 2 | 3 | 4 | 0.758 | 3.97 ± 12.33 | 1.26 ± 0.71 |
| linoleic acid group 5 | 0.020 | 2 | 5 | 3 | 0 | 0.181 | 1 | 2 | 7 | 0 | 0.060 | 1 | 2 | 0 | 7 | 0.137 | 4.36 ± 2.41 | 1.38 ± 0.81 |
| vitamin E group 1 | 6.250 | 7 | 3 | 0 | 0 | 1.000 | 0 | 3 | 7 | 0 | 0.024 * | 0 | 2 | 5 | 3 | 0.754 | 4.33 ± 2.03 | 2.50 ± 1.21 * |
| vitamin E group 2 | 3.125 | 7 | 3 | 0 | 0 | 1.000 | 0 | 4 | 6 | 0 | 0.044 * | 0 | 2 | 4 | 4 | 1.000 | 4.28 ± 1.66 | 2.56 ± 0.97 ** |
| vitamin E group 3 | 1.563 | 6 | 4 | 0 | 0 | 0.673 | 0 | 7 | 3 | 0 | 0.628 | 0 | 3 | 4 | 3 | 0.596 | 3.62 ± 1.55 | 2.59 ± 1.03 ** |
| vitamin E group 4 | 0.781 | 6 | 4 | 0 | 0 | 0.673 | 0 | 7 | 3 | 0 | 0.628 | 0 | 4 | 3 | 3 | 0.475 | 2.92 ± 2.38 | 1.66 ± 1.02 |
| vitamin E group 5 | 0.391 | 8 | 2 | 0 | 0 | 0.660 | 2 | 4 | 3 | 1 | 0.805 | 1 | 4 | 1 | 4 | 0.439 | 3.52 ± 2.12 | 1.90 ± 1.33 |
| oleic acid group 1 | 0.00055 | 3 | 7 | 0 | 0 | 0.081 | 2 | 1 | 5 | 2 | 0.132 | 2 | 0 | 0 | 8 | 0.240 | 4.65 ± 2.34 | 2.05 ± 1.08 |
| oleic acid group 2 | 0.00028 | 1 | 9 | 0 | 0 | 0.058 | 1 | 1 | 5 | 3 | 0.051 | 1 | 1 | 5 | 3 | 0.686 | 4.70 ± 2.39 | 2.14 ± 1.32 |
| oleic acid group 3 | 0.00015 | 3 | 7 | 0 | 0 | 0.081 | 4 | 3 | 3 | 0 | 0.375 | 2 | 4 | 0 | 4 | 0.279 | 3.90 ± 2.20 | 1.91 ± 1.35 |
| oleic acid group 4 | 0.00008 | 3 | 7 | 0 | 0 | 0.081 | 1 | 2 | 4 | 3 | 0.054 | 3 | 1 | 1 | 5 | 0.700 | 3.67 ± 2.25 | 2.30 ± 1.32 |
| oleic acid group 5 | 0.00004 | 8 | 2 | 0 | 0 | 0.897 | 7 | 1 | 2 | 0 | 0.022 * | 5 | 3 | 2 | 0 | 0.001 ** | 2.58 ± 2.36 | 1.66 ± 1.55 |
| Tran squalene group 1 | 0.123 | 7 | 3 | 0 | 0 | 1.000 | 6 | 3 | 1 | 0 | 0.013 * | 6 | 2 | 2 | 0 | 0.002 ** | 1.52 ± 1.73 ** | 0.90 ± 1.10 * |
| Trans squalene group 2 | 0.061 | 2 | 8 | 0 | 0 | 0.064 | 1 | 5 | 2 | 2 | 0.493 | 0 | 2 | 4 | 4 | 1.000 | 4.03 ± 2.06 | 1.72 ± 0.77 |
| Trans squalene group 3 | 0.031 | 3 | 7 | 0 | 0 | 0.081 | 2 | 2 | 4 | 2 | 0.267 | 2 | 0 | 3 | 5 | 0.876 | 4.97 ± 2.34 | 2.27 ± 1.26 |
| Trans squalene group 4 | 0.016 | 1 | 6 | 3 | 0 | 0.840 | 1 | 2 | 4 | 3 | 0.054 | 1 | 2 | 1 | 6 | 0.753 | 4.88 ± 1.44 | 3.03 ± 1.85 |
| Trans squalene group 5 | 0.008 | 2 | 8 | 0 | 0 | 0.064 | 0 | 4 | 3 | 3 | 0.050 | 0 | 2 | 4 | 4 | 1.000 | 4.47 ± 1.38 | 2.29 ± 1.54 |
| Campesterol group 1 | 0.060 | 3 | 7 | 0 | 0 | 0.081 | 2 | 2 | 6 | 0 | 0.330 | 2 | 2 | 1 | 5 | 0.758 | 3.40 ± 2.75 | 1.68 ± 1.59 |
| Campesterol group 2 | 0.030 | 4 | 6 | 0 | 0 | 0.196 | 3 | 2 | 5 | 0 | 0.812 | 3 | 0 | 4 | 3 | 0.438 | 2.96 ± 2.48 | 1.86 ± 0.77 |
| Campesterol group 3 | 0.015 | 1 | 6 | 3 | 0 | 0.840 | 1 | 5 | 3 | 1 | 0.546 | 1 | 2 | 3 | 4 | 0.758 | 3.72 ± 2.45 | 1.42 ± 0.86 |
| Campesterol group 4 | 0.008 | 3 | 6 | 1 | 0 | 0.840 | 2 | 4 | 4 | 0 | 0.868 | 2 | 2 | 1 | 5 | 0.758 | 3.68 ± 2.89 | 1.15 ± 0.97 |
| Campesterol group 5 | 0.004 | 1 | 7 | 2 | 0 | 0.053 | 1 | 3 | 6 | 0 | 0.177 | 1 | 2 | 2 | 5 | 1.000 | 4.14 ± 2.04 | 1.97 ± 0.97 |
| Fucosterol group 1 | 0.123 | 2 | 5 | 3 | 0 | 0.053 | 2 | 2 | 3 | 3 | 0.237 | 1 | 1 | 3 | 5 | 0.815 | 4.32 ± 2.20 | 2.88 ± 1.86 |
| Fucosterol group 2 | 0.061 | 7 | 2 | 1 | 0 | 0.892 | 2 | 4 | 4 | 0 | 0.868 | 2 | 0 | 2 | 6 | 0.049 * | 3.53 ± 2.38 | 2.29 ± 1.53 |
| Fucosterol group 3 | 0.031 | 1 | 4 | 5 | 0 | 0.011 | 0 | 2 | 4 | 4 | 0.002 ** | 0 | 0 | 4 | 6 | 0.037 * | 5.14 ± 1.21 * | 2.83 ± 0.99 |
| Fucosterol group 4 | 0.016 | 5 | 5 | 0 | 0 | 0.412 | 3 | 2 | 5 | 0 | 0.812 | 1 | 3 | 3 | 3 | 0.395 | 4.43 ± 1.56 | 2.59 ± 1.80 |
| Fucosterol group 5 | 0.008 | 2 | 5 | 3 | 0 | 0.053 | 1 | 3 | 4 | 2 | 0.138 | 0 | 3 | 1 | 6 | 0.693 | 4.58 ± 1.70 | 2.64 ± 1.21 |
| Amygdalin group 1 | 0.123 | 0 | 6 | 4 | 0 | 0.055 | 0 | 2 | 4 | 4 | 0.014 * | 0 | 2 | 1 | 7 | 0.034 * | 5.58 ± 1.63 * | 2.29 ± 0.74 |
| Amygdalin group 2 | 0.061 | 7 | 1 | 2 | 0 | 0.384 | 1 | 2 | 4 | 3 | 0.054 | 1 | 1 | 3 | 5 | 0.815 | 5.05 ± 2.00 | 3.46 ± 1.80 |
| Amygdalin group 3 | 0.031 | 2 | 6 | 2 | 0 | 0.376 | 0 | 3 | 3 | 4 | 0.031 * | 0 | 0 | 3 | 7 | 0.029 * | 5.04 ± 1.41 | 2.11 ± 0.97 |
| Amygdalin group 4 | 0.016 | 0 | 8 | 2 | 0 | 0.064 | 0 | 5 | 5 | 0 | 0.178 | 0 | 2 | 3 | 5 | 0.754 | 4.43 ± 1.56 | 2.24 ± 1.28 |
| Amygdalin group 5 | 0.008 | 4 | 4 | 2 | 0 | 0.129 | 2 | 2 | 5 | 1 | 0.298 | 2 | 1 | 4 | 3 | 0.486 | 3.78 ± 2.16 | 1.67 ± 1.23 |
| Group | Dose (mg/cm2/d) | Skin Blackening Time (Day) | Newborn Hair Growth Rating | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 7 | Day 14 | Day 21 | |||||||||||||||
| I | II | III | IV | p | I | II | III | IV | p | I | II | III | IV | p | |||
| Model control group | — | 11.70 ± 3.64 | 8 | 2 | 0 | 0 | — | 1 | 1 | 4 | 4 | — | 0 | 1 | 2 | 7 | — |
| Minoxidi control group | 0.500 | 8.70 ± 1.70 * | 0 | 9 | 1 | 0 | p ≤ 0.01 | 0 | 0 | 0 | 10 | p ≤ 0.05 | 0 | 0 | 0 | 10 | p ≥ 0.05 |
| β-sitosterol group 2 | 0.061 | 8.80 ± 2.04 * | 3 | 5 | 2 | 0 | p ≤ 0.01 | 0 | 0 | 1 | 9 | p ≤ 0.05 | 0 | 0 | 0 | 10 | p ≥ 0.05 |
| β-sitosterol group 3 | 0.031 | 9.10 ± 1.73 | 2 | 8 | 0 | 0 | p ≤ 0.01 | 0 | 0 | 2 | 8 | p ≥ 0.05 | 0 | 0 | 0 | 10 | p ≥ 0.05 |
| β-sitosterol group 4 | 0.016 | 8.80 ± 1.03 * | 1 | 8 | 1 | 0 | p ≤ 0.01 | 0 | 0 | 1 | 9 | p ≤ 0.05 | 0 | 0 | 0 | 10 | p ≥ 0.05 |
| linoleic acid group 1 | 0.313 | 9.10 ± 1.52 | 3 | 6 | 1 | 0 | p ≤ 0.01 | 0 | 0 | 2 | 8 | p ≥ 0.05 | 0 | 0 | 0 | 10 | p ≥ 0.05 |
| linoleic acid group 2 | 0.156 | 8.70 ± 0.82 * | 2 | 8 | 0 | 0 | p ≤ 0.01 | 0 | 0 | 1 | 9 | p ≤ 0.05 | 0 | 0 | 0 | 10 | p ≥ 0.05 |
| linoleic acid group 3 | 0.078 | 9.10 ± 1.29 | 2 | 7 | 1 | 0 | p ≤ 0.01 | 0 | 1 | 3 | 6 | p ≥ 0.05 | 0 | 0 | 0 | 10 | p ≥ 0.05 |
| vitamin E group 1 | 0.313 | 10.20 ± 2.62 | 3 | 7 | 0 | 0 | p ≤ 0.01 | 0 | 2 | 1 | 7 | p ≥ 0.05 | 0 | 0 | 0 | 10 | p ≥ 0.05 |
| vitamin E group 2 | 0.156 | 8.50 ± 1.18 * | 2 | 7 | 1 | 0 | p ≤ 0.01 | 0 | 0 | 1 | 9 | p ≤ 0.05 | 0 | 0 | 0 | 10 | p ≥ 0.05 |
| vitamin E group 3 | 0.078 | 9.10 ± 1.79 | 3 | 7 | 0 | 0 | p ≤ 0.01 | 0 | 1 | 1 | 8 | p ≥ 0.05 | 0 | 0 | 0 | 10 | p ≥ 0.05 |
| Fucosterol group 2 | 0.061 | 9.10 ± 1.10 * | 1 | 9 | 0 | 0 | p ≤ 0.01 | 0 | 1 | 3 | 6 | p ≥ 0.05 | 0 | 0 | 0 | 10 | p ≥ 0.05 |
| Fucosterol group 3 | 0.031 | 10.20 ± 2.62 | 5 | 5 | 0 | 0 | p ≥ 0.05 | 1 | 0 | 4 | 5 | p ≥ 0.05 | 0 | 0 | 0 | 10 | p ≥ 0.05 |
| Fucosterol group 4 | 0.016 | 9.20 ± 2.49 | 2 | 7 | 1 | 0 | p ≤ 0.01 | 0 | 1 | 2 | 7 | p ≥ 0.05 | 0 | 0 | 0 | 10 | p ≥ 0.05 |
| Amygdalin group 2 | 0.061 | 9.70 ± 1.83 | 0 | 10 | 0 | 0 | p ≤ 0.01 | 0 | 0 | 2 | 8 | p ≥ 0.05 | 0 | 0 | 0 | 10 | p ≥ 0.05 |
| Amygdalin group 3 | 0.031 | 9.40 ± 1.43 | 3 | 7 | 0 | 0 | p ≤ 0.01 | 0 | 1 | 2 | 7 | p ≥ 0.05 | 0 | 0 | 1 | 9 | p ≥ 0.05 |
| Amygdalin group 4 | 0.016 | 9.20 ± 0.92 | 2 | 7 | 1 | 0 | p ≤ 0.01 | 0 | 1 | 4 | 5 | p ≥ 0.05 | 0 | 0 | 0 | 10 | p ≥ 0.05 |
| Group | Dose (mg/cm2/d) | Number of Animals (Number) | Newborn Hair Length (Day 7, cm) | Newborn Hair Length (Day 14, cm) | Newborn Hair Weight (g) |
|---|---|---|---|---|---|
| Model control group | — | 10 | 0.138 ± 0.151 | 0.625 ± 0.186 | 0.00154 ± 0.00071 |
| Minoxidi control group | 0.500 | 10 | 0.420 ± 0.133 ** | 0.816 ± 0.161 ** | 0.00260 ± 0.00035 ** |
| β-sitosterol group 2 | 0.061 | 10 | 0.363 ± 0.203 ** | 0.798 ± 0.116 ** | 0.00284 ± 0.00044 ** |
| β-sitosterol group 3 | 0.031 | 10 | 0.336 ± 0.153 ** | 0.740 ± 0.107 | 0.00258 ± 0.00037 ** |
| β-sitosterol group 4 | 0.016 | 10 | 0.373 ± 0.113 ** | 0.786 ± 0.120 ** | 0.00299 ± 0.00051 ** |
| linoleic acid group 1 | 0.313 | 10 | 0.296 ± 0.124 ** | 0.693 ± 0.071 | 0.00277 ± 0.00037 ** |
| linoleic acid group 2 | 0.156 | 10 | 0.297 ± 0.152 ** | 0.739 ± 0.152 | 0.00230 ± 0.00050 ** |
| linoleic acid group 3 | 0.078 | 10 | 0.238 ± 0.188 | 0.787 ± 0.138 ** | 0.00243 ± 0.00053 ** |
| vitamin E group 1 | 0.313 | 10 | 0.238 ± 0.173 | 0.693 ± 0.156 | 0.00249 ± 0.00029 ** |
| vitamin E group 2 | 0.156 | 10 | 0.316 ± 0.167 ** | 0.716 ± 0.131 | 0.00242 ± 0.00028 ** |
| vitamin E group 3 | 0.078 | 10 | 0.314 ± 0.064 ** | 0.755 ± 0.065 | 0.00261 ± 0.00063 ** |
| Fucosterol group 2 | 0.061 | 10 | 0.296 ± 0.145 ** | 0.762 ± 0.131 | 0.00260 ± 0.00050 ** |
| Fucosterol group 3 | 0.031 | 10 | 0.186 ± 0.173 | 0.735 ± 0.129 | 0.00272 ± 0.00056 ** |
| Fucosterol group 4 | 0.016 | 10 | 0.252 ± 0.155 | 0.747 ± 0.087 | 0.00257 ± 0.00041 ** |
| Amygdalin group 2 | 0.061 | 10 | 0.362 ± 0.101 ** | 0.717 ± 0.093 | 0.00216 ± 0.00041 ** |
| Amygdalin group 3 | 0.031 | 10 | 0.201 ± 0.146 | 0.619 ± 0.182 | 0.00208 ± 0.00056 ** |
| Amygdalin group 4 | 0.016 | 10 | 0.208 ± 0.121 | 0.693 ± 0.100 | 0.00250 ± 0.00058 |
| Group | Dose (mg/cm2/d) | Dermis Thickness (nm) | Dermis Thickness (Number) | Secondary Hair Follicle (Number) | Total Number of Hair Follicles (Number) |
|---|---|---|---|---|---|
| Model control group | — | 360.15 ± 150.44 | 22.17 ±12.25 | 36.33 ± 21.02 | 59.7 ± 29.26 |
| Minoxidi control group | 0.50 | 344.63 ± 137.09 | 20.10 ± 12.98 | 43.37 ± 31.69 | 63.47 ± 44.28 |
| β-sitosterol group 2 | 0.061 | 345.24 ± 107.93 | 19.30 ± 11.15 | 41.17 ± 27.59 | 60.47 ± 38.15 |
| linoleic acid group 2 | 0.156 | 381.06 ± 156.68 | 14.27 ± 10.18 | 24.83 ± 21.15 | 39.10 ± 32.96 |
| vitamin E group 2 | 0.156 | 350.47 ± 150.87 | 20.63 ± 11.85 | 37.73 ± 22.60 | 58.37 ± 34.36 |
| Group | Dose (mg/cm2/d) | β-catenin | GSK3β | Cyclin D 1 | LEF 1 |
|---|---|---|---|---|---|
| Model control group | — | 0.23 ± 0.11 | 0.36 ± 0.16 | 1.61 ± 0.68 | 0.43 ± 0.17 |
| Minoxidi control group | 0.50 | 0.71 ± 0.27 ** | 0.79 ± 0.27 ** | 1.07 ± 0.21 * | 0.81 ± 0.20 ** |
| β-sitosterol group 2 | 0.061 | 0.27 ± 0.26 | 0.57 ± 0.18 ** | 1.31 ± 0.28 | 0.61 ± 0.18 * |
| linoleic acid group 2 | 0.156 | 0.39 ± 0.26 | 0.72 ± 0.26 ** | 1.41 ± 0.26 | 0.70 ± 0.32 * |
| vitamin E group 2 | 0.156 | 0.50 ± 0.50 | 0.73 ± 0.19 ** | 1.21 ± 0.43 | 0.91 ± 0.49 ** |
| Group | Dose (mg/cm2/d) | Number of Animals (Number) | β-catenin | GSK3β | Cyclin D 1 | LEF 1 |
|---|---|---|---|---|---|---|
| Model control group | — | 10 | 0.222 ± 0.008 | 0.290 ± 0.018 | 0.195 ± 0.025 | 0.242 ± 0.010 |
| Minoxidi control group | 0.50 | 10 | 0.220 ± 0.010 | 0.278 ± 0.011 | 0.188 ± 0.028 | 0.241 ± 0.012 |
| β-sitosterol group 2 | 0.061 | 10 | 0.218 ± 0.006 | 0.284 ± 0.022 | 0.183 ± 0.031 | 0.231 ± 0.015 |
| linoleic acid group 2 | 0.156 | 10 | 0.217 ± 0.008 | 0.278 ± 0.009 | 0.171 ± 0.021 | 0.229 ± 0.016 |
| vitamin E group 2 | 0.156 | 10 | 0.216 ± 0.006 | 0.279 ± 0.012 | 0.192 ± 0.030 | 0.232 ± 0.017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Zhang, J.; Chen, W.; Li, X.; Fu, K.; Sun, W.; Liang, Y.; Xu, M.; Zhang, J.; Fan, G.; et al. Identification of Hair Growth Promoting Components in the Kernels of Prunus mira Koehne and Their Mechanism of Action. Molecules 2022, 27, 5242. https://doi.org/10.3390/molecules27165242
Zhou Y, Zhang J, Chen W, Li X, Fu K, Sun W, Liang Y, Xu M, Zhang J, Fan G, et al. Identification of Hair Growth Promoting Components in the Kernels of Prunus mira Koehne and Their Mechanism of Action. Molecules. 2022; 27(16):5242. https://doi.org/10.3390/molecules27165242
Chicago/Turabian StyleZhou, You, Jingwen Zhang, Wanyue Chen, Xiaoli Li, Ke Fu, Weijun Sun, Yuan Liang, Min Xu, Jing Zhang, Gang Fan, and et al. 2022. "Identification of Hair Growth Promoting Components in the Kernels of Prunus mira Koehne and Their Mechanism of Action" Molecules 27, no. 16: 5242. https://doi.org/10.3390/molecules27165242
APA StyleZhou, Y., Zhang, J., Chen, W., Li, X., Fu, K., Sun, W., Liang, Y., Xu, M., Zhang, J., Fan, G., Yin, H., & Wang, Z. (2022). Identification of Hair Growth Promoting Components in the Kernels of Prunus mira Koehne and Their Mechanism of Action. Molecules, 27(16), 5242. https://doi.org/10.3390/molecules27165242