Impact of Tralopyril and Triazolyl Glycosylated Chalcone in Human Retinal Cells’ Lipidome
Abstract
:1. Introduction
2. Results and Discussion
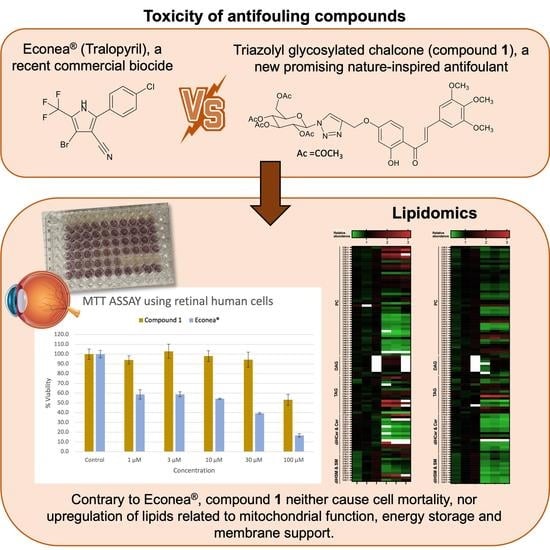
2.1. Cytotoxicity of Econea® and Compound 1 in Retinal Human Cells
2.2. Effects of Acute Exposure on Cellular Lipidome
3. Materials and Methods
3.1. Synthesis
3.2. Cell Culture
3.3. Mitochondrial Viability Assay
3.4. Lipid Extraction
3.5. Sample Normalization
3.6. Sample Normalization of Liquid Chromatography–Tandem Mass Spectrometry Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- de Campos, B.G.; Figueiredo, J.; Perina, F.; Abessa, D.M.d.S.; Loureiro, S.; Martins, R. Occurrence, effects and environmental risk of antifouling biocides (EU PT21): Are marine ecosystems threatened? Crit. Rev. Environ. Sci. Technol. 2021, 52, 3179–3210. [Google Scholar] [CrossRef]
- Schultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic impact of biofouling on a naval surface ship. Biofouling 2011, 27, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Batista-Andrade, J.A.; Caldas, S.S.; Batista, R.M.; Castro, I.B.; Fillmann, G.; Primel, E.G. From TBT to booster biocides: Levels and impacts of antifouling along coastal areas of Panama. Environ. Pollut. 2018, 234, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Muller-Karanassos, C.; Arundel, W.; Lindeque, P.K.; Vance, T.; Turner, A.; Cole, M. Environmental concentrations of antifouling paint particles are toxic to sediment-dwelling invertebrates. Environ. Pollut. 2021, 268, 115754. [Google Scholar] [CrossRef] [PubMed]
- Amara, I.; Miled, W.; Slama, R.B.; Ladhari, N. Antifouling processes and toxicity effects of antifouling paints on marine environment. A review. Environ. Toxicol. Pharmacol. 2018, 57, 115–130. [Google Scholar] [CrossRef]
- Chen, X.; Teng, M.; Zhang, J.; Qian, L.; Duan, M.; Cheng, Y.; Zhao, F.; Zheng, J.; Wang, C. Tralopyril induces developmental toxicity in zebrafish embryo (Danio rerio) by disrupting the thyroid system and metabolism. Sci. Total Environ. 2020, 746, 141860. [Google Scholar] [CrossRef]
- Thomas, K.V.; Brooks, S. The environmental fate and effects of antifouling paint biocides. Biofouling 2010, 26, 73–88. [Google Scholar] [CrossRef]
- Oliveira, I.B.; Groh, K.J.; Stadnicka-Michalak, J.; Schönenberger, R.; Beiras, R.; Barroso, C.M.; Langford, K.H.; Thomas, K.V.; Suter, M.J.F. Tralopyril bioconcentration and effects on the gill proteome of the Mediterranean mussel Mytilus galloprovincialis. Aquat. Toxicol. 2016, 177, 198–210. [Google Scholar] [CrossRef]
- Oliveira, I.B.; Beiras, R.; Thomas, K.V.; Suter, M.J.F.; Barroso, C.M. Acute toxicity of tralopyril, capsaicin and triphenylborane pyridine to marine invertebrates. Ecotoxicology 2014, 23, 1336–1344. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, J.; Teng, M.; Zhang, J.; Qian, L.; Duan, M.; Cheng, Y.; Zhao, W.; Wang, Z.; Wang, C. Tralopyril affects locomotor activity of zebrafish (Danio rerio) by impairing tail muscle tissue, the nervous system, and energy metabolism. Chemosphere 2022, 286, 131866. [Google Scholar] [CrossRef]
- Guardiola, F.A.; Cuesta, A.; Meseguer, J.; Esteban, M.A. Risks of Using Antifouling Biocides in Aquaculture. Int. J. Mol. Sci. 2012, 13, 1541–1560. [Google Scholar] [CrossRef] [PubMed]
- Links, I.; Van Der Jagt, K.E.; Christopher, Y.; Lurvink, M.; Schinkel, J.; Tielemans, E.; Van Hemmen, J.J. Occupational exposure during application and removal of antifouling paints. Ann. Occup. Hyg. 2007, 51, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Vilas-Boas, C.; Carvalhal, F.; Pereira, B.; Carvalho, S.; Sousa, E.; Pinto, M.M.M.; Calhorda, M.J.; Vasconcelos, V.; Almeida, J.R.; Silva, E.R.; et al. One Step Forward towards the Development of Eco-Friendly Antifouling Coatings: Immobilization of a Sulfated Marine-Inspired Compound. Mar. Drugs 2020, 18, 489. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.; Correia-da-Silva, M.; Sousa, E.; Antunes, J.; Pinto, M.; Vasconcelos, V.; Cunha, I. Antifouling potential of nature-inspired sulfated compounds. Sci. Rep. 2017, 7, 42424. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.; Palmeira, A.; Campos, A.; Cunha, I.; Freitas, M.; Felpeto, A.B.; Turkina, M.V.; Vasconcelos, V.; Pinto, M.; Correia-da-Silva, M.; et al. Structure-Antifouling Activity Relationship and Molecular Targets of Bio-Inspired(thio)xanthones. Biomolecules 2020, 10, 1126. [Google Scholar] [CrossRef]
- Neves, A.R.; Almeida, J.R.; Carvalhal, F.; Câmara, A.; Pereira, S.; Antunes, J.; Vasconcelos, V.; Pinto, M.; Silva, E.R.; Sousa, E.; et al. Overcoming environmental problems of biocides: Synthetic bile acid derivatives as a sustainable alternative. Ecotoxicol. Environ. Saf. 2020, 187, 109812. [Google Scholar] [CrossRef]
- Vilas-Boas, C.; Neves, A.R.; Carvalhal, F.; Pereira, S.; Calhorda, M.J.; Vasconcelos, V.; Pinto, M.; Sousa, E.; Almeida, J.R.; Silva, E.R.; et al. Multidimensional characterization of a new antifouling xanthone: Structure-activity relationship, environmental compatibility, and immobilization in marine coatings. Ecotoxicol. Environ. Saf. 2021, 228, 112970. [Google Scholar] [CrossRef]
- Rita Neves, A.; Vilas Boas, C.; Gonçalves, C.; Vasconcelos, V.; Pinto, M.; Silva, E.R.; Sousa, E.; Almeida, J.R.; Correia-da-Silva, M. Gallic acid derivatives as inhibitors of mussel (Mytilus galloprovincialis) larval settlement: Lead optimization, biological evaluation and use in antifouling coatings. Bioorg. Chem. 2022, 126, 105911. [Google Scholar] [CrossRef]
- Pereira, D.; Gonçalves, C.; Martins, B.T.; Palmeira, A.; Vasconcelos, V.; Pinto, M.; Almeida, J.R.; Correia-da-Silva, M.; Cidade, H. Flavonoid Glycosides with a Triazole Moiety for Marine Antifouling Applications: Synthesis and Biological Activity Evaluation. Marine Drugs 2021, 19, 5. [Google Scholar] [CrossRef]
- Chen, L.; Qian, P.-Y. Review on Molecular Mechanisms of Antifouling Compounds: An Update since 2012. Mar. Drugs 2017, 15, 264. [Google Scholar] [CrossRef]
- Yamamoto, I. [Pollution of fish and shellfish with organotin compounds and estimation of daily intake]. Hokkaido Igaku Zasshi 1994, 69, 273–281. [Google Scholar] [PubMed]
- Dreier, D.A.; Nouri, M.Z.; Denslow, N.D.; Martyniuk, C.J. Lipidomics reveals multiple stressor effects (temperature × mitochondrial toxicant) in the zebrafish embryo toxicity test. Chemosphere 2021, 264, 128472. [Google Scholar] [CrossRef] [PubMed]
- Dreier, D.A.; Bowden, J.A.; Aristizabal-Henao, J.J.; Denslow, N.D.; Martyniuk, C.J. Ecotoxico-lipidomics: An emerging concept to understand chemical-metabolic relationships in comparative fish models. Comp. Biochem. Physiol. Part D Genom. Proteom. 2020, 36, 100742. [Google Scholar] [CrossRef] [PubMed]
- Matich, E.K.; Chavez Soria, N.G.; Aga, D.S.; Atilla-Gokcumen, G.E. Applications of metabolomics in assessing ecological effects of emerging contaminants and pollutants on plants. J. Hazard. Mater. 2019, 373, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Chavez Soria, N.G.; Aga, D.S.; Atilla-Gokcumen, G.E. Lipidomics reveals insights on the biological effects of copper oxide nanoparticles in a human colon carcinoma cell line. Mol. Omics 2019, 15, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Atilla-Gokcumen, G.E.; Muro, E.; Relat-Goberna, J.; Sasse, S.; Bedigian, A.; Coughlin, M.L.; Garcia-Manyes, S.; Eggert, U.S. Dividing cells regulate their lipid composition and localization. Cell 2014, 156, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Benov, L. Effect of growth media on the MTT colorimetric assay in bacteria. PLoS ONE 2019, 14, e0219713. [Google Scholar] [CrossRef]
- Vajrabhaya, L.-o.; Korsuwannawong, S. Cytotoxicity evaluation of a Thai herb using tetrazolium (MTT) and sulforhodamine B (SRB) assays. J. Anal. Sci. Technol. 2018, 9, 15. [Google Scholar] [CrossRef]
- Tang, C.H.; Lin, C.Y.; Sun, P.P.; Lee, S.H.; Wang, W.H. Modeling the effects of Irgarol 1051 on coral using lipidomic methodology for environmental monitoring and assessment. Sci. Total Environ. 2018, 627, 571–578. [Google Scholar] [CrossRef]
- Mnif, W.; Hassine, A.I.H.; Bouaziz, A.; Bartegi, A.; Thomas, O.; Roig, B. Effect of endocrine disruptor pesticides: A review. Int. J. Environ. Res. Public Health 2011, 8, 2265–2303. [Google Scholar] [CrossRef]
- del Solar, V.; Lizardo, D.Y.; Li, N.; Hurst, J.J.; Brais, C.J.; Atilla-Gokcumen, G.E. Differential Regulation of Specific Sphingolipids in Colon Cancer Cells during Staurosporine-Induced Apoptosis. Chem. Biol. 2015, 22, 1662–1670. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lizardo, D.Y.; Atilla-Gokcumen, G.E. Specific Triacylglycerols Accumulate via Increased Lipogenesis During 5-FU-Induced Apoptosis. ACS Chem. Biol. 2016, 11, 2583–2587. [Google Scholar] [CrossRef] [PubMed]
- Brovkovych, V.; Izhar, Y.; Danes, J.M.; Dubrovskyi, O.; Sakallioglu, I.T.; Morrow, L.M.; Atilla-Gokcumen, G.E.; Frasor, J. Fatostatin induces pro- and anti-apoptotic lipid accumulation in breast cancer. Oncogenesis 2018, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Bartke, N.; Hannun, Y.A. Bioactive sphingolipids: Metabolism and function. J. Lipid Res. 2009, 50, S91–S96. [Google Scholar] [CrossRef]
- Jarc, E.; Petan, T. Lipid Droplets and the Management of Cellular Stress. Yale J. Biol. Med. 2019, 92, 435–452. [Google Scholar]
- van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta (BBA) Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef]
- Lizardo, D.Y.; Parisi, L.R.; Li, N.; Atilla-Gokcumen, G.E. Noncanonical Roles of Lipids in Different Cellular Fates. Biochemistry 2018, 57, 22–29. [Google Scholar] [CrossRef]
- Hwang, S.; Williams, J.F.; Kneissig, M.; Lioudyno, M.; Rivera, I.; Helguera, P.; Busciglio, J.; Storchova, Z.; King, M.C.; Torres, E.M. Suppressing Aneuploidy-Associated Phenotypes Improves the Fitness of Trisomy 21 Cells. Cell Rep. 2019, 29, 2473–2488.e2475. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, J.; Teng, M.; Zhang, J.; Qian, L.; Duan, M.; Wang, Z.; Wang, C. Environmentally relevant concentrations of tralopyril affect carbohydrate metabolism and lipid metabolism of zebrafish (Danio rerio) by disrupting mitochondrial function. Ecotoxicol. Environ. Saf. 2021, 223, 112615. [Google Scholar] [CrossRef]
- Li, N.; Sancak, Y.; Frasor, J.; Atilla-Gokcumen, G.E. A Protective Role for Triacylglycerols during Apoptosis. Biochemistry 2018, 57, 72–80. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilas-Boas, C.; Running, L.; Pereira, D.; Cidade, H.; Correia-da-Silva, M.; Atilla-Gokcumen, G.E.; Aga, D.S. Impact of Tralopyril and Triazolyl Glycosylated Chalcone in Human Retinal Cells’ Lipidome. Molecules 2022, 27, 5247. https://doi.org/10.3390/molecules27165247
Vilas-Boas C, Running L, Pereira D, Cidade H, Correia-da-Silva M, Atilla-Gokcumen GE, Aga DS. Impact of Tralopyril and Triazolyl Glycosylated Chalcone in Human Retinal Cells’ Lipidome. Molecules. 2022; 27(16):5247. https://doi.org/10.3390/molecules27165247
Chicago/Turabian StyleVilas-Boas, Cátia, Logan Running, Daniela Pereira, Honorina Cidade, Marta Correia-da-Silva, Gunes Ekin Atilla-Gokcumen, and Diana S. Aga. 2022. "Impact of Tralopyril and Triazolyl Glycosylated Chalcone in Human Retinal Cells’ Lipidome" Molecules 27, no. 16: 5247. https://doi.org/10.3390/molecules27165247
APA StyleVilas-Boas, C., Running, L., Pereira, D., Cidade, H., Correia-da-Silva, M., Atilla-Gokcumen, G. E., & Aga, D. S. (2022). Impact of Tralopyril and Triazolyl Glycosylated Chalcone in Human Retinal Cells’ Lipidome. Molecules, 27(16), 5247. https://doi.org/10.3390/molecules27165247