Dynamic Changes in Anthocyanin Accumulation and Cellular Antioxidant Activities in Two Varieties of Grape Berries during Fruit Maturation under Different Climates
Abstract
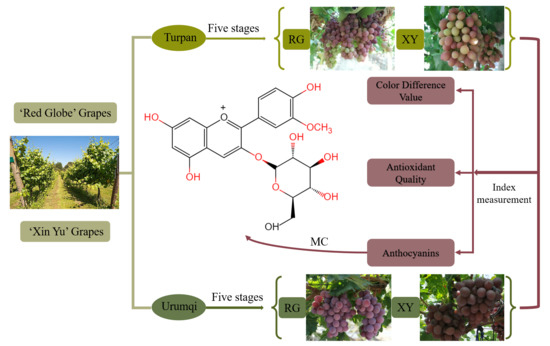
:1. Introduction
2. Results and Discussion
2.1. Climatic Conditions in Urumqi and Turpan
2.2. Color Difference Values of Grape Berries in Urumqi and Turpan
2.3. Gene Expression of Grape Berries in Urumqi and Turpan
2.4. Phenolic Compounds, such as the Anthocyanins, of Grape Berries in Urumqi and Turpan
2.5. Antioxidant Activities of Grape Berries in Urumqi and Turpan
2.6. Cytotoxicity and the Anti-Proliferative Activities of Grape Berries in Urumqi and Turpan
2.7. Correlation Analysis
2.8. Principal Component Analysis (PCA) and Cluster Analysis in Grape Berries
3. Materials and Methods
3.1. Materials
3.2. Reagent and Chemicals
3.3. Determination of Color Difference Value
3.4. Total RNA Extraction, Reverse Transcription and RT-qPCR Analysis
3.5. Phenolic Substance Extracts of Grape Berries
3.6. Determination of Phenolic Substances, Such as Anthocyanins
3.7. Determination of Antioxidant Activities
3.8. Determination of Cytotoxicity Assay and Anti-Proliferation Activity
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, K.; Maltese, F.; Choi, Y.H.; Verpoorte, R. Metabolic constituents of grapevine and grape-derived products. Phytochem. Rev. 2010, 9, 357–378. [Google Scholar] [CrossRef] [Green Version]
- Van den Ende, W.; El-Esawe, S.K. Sucrose signaling pathways leading to fructan and anthocyanin accumulation: A dual function in abiotic and biotic stress responses? Environ. Exp. Bot. 2014, 108, 4–13. [Google Scholar] [CrossRef]
- Li, X.; Ouyang, X.F.; Zhang, Z.S.; He, L.; Wang, Y.; Li, Y.H.; Zhao, J.; Chen, Z.; Wang, C.N.; Ding, L.L.; et al. Over-expression of the red plant gene r1 enhances anthocyanin production and resistance to bollworm and spider mite in cotton. Mol. Genet. Genom. 2019, 294, 469–478. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of oxidative and drought tolerance in arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef]
- Zhang, T.J.; Chow, W.S.; Liu, X.T.; Zhang, P.; Liu, N.; Peng, C.L. A magic red coat on the surface of young leaves: Anthocyanins distributed in trichome layer protect castanopsis fissa leaves from photoinhibition. Tree Physiol. 2016, 36, 1296–1306. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Yeo, J. Bioactivities of phenolics by focusing on suppression of chronic diseases: A review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef] [Green Version]
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural practice and environmental impacts on the flavonoid composition of grapes and wine: A review of recent research. Am. J. Enol. Vitic. 2006, 57, 257–268. [Google Scholar]
- He, F.; Mu, L.; Yan, G.L.; Liang, N.N.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Biosynthesis of anthocyanins and their regulation in colored grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.Y.; Song, C.Z.; Chi, M.; Wang, T.M.; Zuo, L.L.; Li, X.L.; Zhang, Z.W.; Xi, Z.M. The effects of light and ethylene and their interaction on the regulation of proanthocyanidin and anthocyanin synthesis in the skins of vitis vinifera berries. Plant Growth Regul. 2016, 79, 377–390. [Google Scholar] [CrossRef]
- Cohen, S.D.; Tarara, J.M.; Kennedy, J.A. Assessing the impact of temperature on grape phenolic metabolism. Anal. Chim. Acta 2008, 621, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Gaiotti, F.; Pastore, C.; Filippetti, I.; Lovat, L.; Belfiore, N.; Tomasi, D. Low night temperature at veraison enhances the accumulation of anthocyanins in corvina grapes (Vitis vinifera L.). Sci. Rep. 2018, 8, 8719. [Google Scholar] [CrossRef] [Green Version]
- Koshita, Y.; Yamane, T.; Yakushiji, H.; Azuma, A.; Mitani, N. Regulation of skin color in ‘aki queen’ grapes: Interactive effects of temperature, girdling, and leaf shading treatments on coloration and total soluble solids. Sci. Hortic. 2011, 129, 98–101. [Google Scholar] [CrossRef]
- Mori, K.; Goto-Yamamoto, N.; Kitayama, M.; Hashizume, K. Loss of anthocyanins in red-wine grape under high temperature. J. Exp. Bot. 2007, 58, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Gamboa, G.; Zheng, W.; Martinez de Toda, F. Current viticultural techniques to mitigate the effects of global warming on grape and wine quality: A comprehensive review. Food Res. Int. 2021, 139, 109946. [Google Scholar] [CrossRef]
- Islam, M.Z.; Lee, Y.T.; Mele, M.A.; Choi, I.L.; Kang, H.M. The effect of phosphorus and root zone temperature on anthocyanin of red romaine lettuce. Agronomy 2019, 9, 47. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.H.; Li, W.F.; Mao, J.; Li, W.; Zuo, C.W.; Zhao, X.; Dawuda, M.M.; Shi, X.Y.; Chen, B.H. Synthesis of light-inducible and light-independent anthocyanins regulated by specific genes in grape ‘marselan’ (V. vinifera L.). PeerJ 2019, 7, e6521. [Google Scholar] [CrossRef] [Green Version]
- Zha, Q.; Xi, X.J.; He, Y.; Jiang, A.L. Bagging affecting sugar and anthocyanin metabolism in the ripening period of grape berries. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 1194–1205. [Google Scholar] [CrossRef] [Green Version]
- Mori, K.; Sugaya, S.; Gemma, H. Decreased anthocyanin biosynthesis in grape berries grown under elevated night temperature condition. Sci. Hortic. 2005, 105, 319–330. [Google Scholar] [CrossRef]
- Gonzalez, M.; Salazar, E.; Cabrera, S.; Olea, P.; Carrasco, B. Analysis of anthocyanin biosynthesis genes expression profiles in contrasting cultivars of japanese plum (Prunus salicina L.) during fruit development. Gene Expr. Patterns 2016, 21, 54–62. [Google Scholar] [CrossRef]
- Poudel, P.R.; Mochioka, R.; Beppu, K.; Kataoka, I. Influence of temperature on berry composition of interspecific hybrid wine grape ‘kadainou r-1’ (Vitis ficifolia var. Ganebu x V. vinifera ‘muscat of alexandria’). J. Jpn. Soc. Hortic. Sci. 2009, 78, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Gouot, J.C.; Smith, J.P.; Holzapfel, B.P.; Walker, A.R.; Barril, C. Grape berry flavonoids: A review of their biochemical responses to high and extreme high temperatures. J. Exp. Bot. 2019, 70, 397–423. [Google Scholar] [CrossRef]
- de Rosas, I.; Ponce, M.T.; Malovini, E.; Deis, L.; Cavagnaro, B.; Cavagnaro, P. Loss of anthocyanins and modification of the anthocyanin profiles in grape berries of malbec and bonarda grown under high temperature conditions. Plant Sci. 2017, 258, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Gao-Takai, M.; Katayama-Ikegami, A.; Matsuda, K.; Shindo, H.; Uemae, S.; Oyaizu, M. A low temperature promotes anthocyanin biosynthesis but does not accelerate endogenous abscisic acid accumulation in red-skinned grapes. Plant Sci. 2019, 283, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, J.; Dokoozlian, N.; Ebisuda, N. Sunlight exposure and temperature effects on berry growth and composition of cabernet sauvignon and grenache in the central san joaquin valley of california. Am. J. Enol. Vitic. 2001, 52, 1–7. [Google Scholar]
- Spayd, S.E.; Tarara, J.M.; Mee, D.L.; Ferguson, J.C. Separation of sunlight and temperature effects on the composition of vitis vinifera cv. Merlot berries. Am. J. Enol. Vitic. 2002, 53, 171–182. [Google Scholar]
- Serio, S.; Rivero-Perez, M.D.; Correia, A.C.; Jordao, A.M.; Gonzalez-San Jose, M.L. Analysis of commercial grape raisins: Phenolic content, antioxidant capacity and radical scavenger activity. Cienc. Tec. Vitivinic. 2014, 29, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kallithraka, S.; Aliaj, L.; Makris, D.P.; Kefalas, P. Anthocyanin profiles of major red grape (vitis vinifera l.) varieties cultivated in greece and their relationship with in vitro antioxidant characteristics. Int. J. Food Sci. Technol. 2009, 44, 2385–2393. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (caa) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
- Wang, H.L.; Guo, X.B.; Hu, X.D.; Li, T.; Fu, X.; Liu, R.H. Comparison of phytochemical profiles, antioxidant and cellular antioxidant activities of different varieties of blueberry (Vaccinium spp.). Food Chem. 2017, 217, 773–781. [Google Scholar] [CrossRef]
- Zhou, K.; Raffoul, J.J. Potential anticancer properties of grape antioxidants. J. Oncol. 2012, 2012, 803294. [Google Scholar] [CrossRef] [Green Version]
- Somasagara, R.R.; Hegde, M.; Chiruvella, K.K.; Musini, A.; Choudhary, B.; Raghavan, S.C. Extracts of strawberry fruits induce intrinsic pathway of apoptosis in breast cancer cells and inhibits tumor progression in mice. PLoS ONE 2012, 7, e47021. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Chang, X.X.; Wang, H.; Brennan, C.S.; Guo, X.B. Phytochemicals accumulation in sanhua plum (Prunus salicina L.) during fruit development and their potential use as antioxidants. J. Agric. Food Chem. 2019, 67, 2459–2466. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.X.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Felice, D.L.; Sun, J.; Liu, R.H. A modified methylene blue assay for accurate cell counting. J. Funct. Food. 2009, 1, 109–118. [Google Scholar] [CrossRef]
- Yang, J.; Liu, R.H. Synergistic effect of apple extracts and quercetin 3-beta-d-glucoside combination on antiproliferative activity in mcf-7 human breast cancer cells in vitro. J. Agric. Food Chem. 2009, 57, 8581–8586. [Google Scholar] [CrossRef]




| Stage | Groups | C mg/100 g FW | FA mg/100 g FW | EC mg/100 g FW | PB1 mg/100 g FW | PB2 mg/100 g FW | Dp μg/g FW | Cy μg/g FW | Pt μg/g FW | Pn μg/g FW | Mv μg/g FW |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | RG−U | 26.50 ± 1.97e | 13.28 ± 0.20b | 16.36 ± 0.62e | 6.30 ± 0.19e | 3.50 ± 0.02e | ND | ND | ND | ND | ND |
| RG−T | 24.68 ± 0.79f | 9.20 ± 0.23d | 21.87 ± 0.35b | 6.18 ± 0.15e | 3.91 ± 0.01d | ND | ND | ND | ND | ND | |
| XY−U | 45.32 ± 1.05a | 14.92 ± 0.31a | 14.11 ± 0.21f | 9.91 ± 0.30a | 6.69 ± 0.07a | ND | ND | ND | ND | ND | |
| XY−T | 40.24 ± 0.54b | 13.20 ± 0.11b | 17.94 ± 2.02d | 9.58 ± 0.01b | 4.41 ± 0.10c | ND | 0.02 ± 0.00l | ND | 0.47 ± 0.01n | ND | |
| S2 | RG−U | 24.37 ± 0.31fg | 8.37 ± 0.06f | 27.23 ± 0.43a | 6.69 ± 0.20d | 3.95 ± 0.03d | ND | 1.27 ± 0.07f | ND | 5.11 ± 0.27i | ND |
| RG−T | 16.69 ± 0.27h | 5.29 ± 0.01h | 13.81 ± 0.12f | 4.00 ± 0.01j | 1.78 ± 0.00jk | ND | 0.12 ± 0.01k | ND | 1.07 ± 0.07m | ND | |
| XY−U | 34.10 ± 0.47d | 12.03 ± 0.07c | 21.06 ± 0.23b | 8.92 ± 0.23c | 5.37 ± 0.03b | 0.06 ± 0.00g | 0.54 ± 0.02i | ND | 5.16 ± 0.20i | 0.54 ± 0.02i | |
| XY−T | 25.17 ± 0.19f | 6.05 ± 0.05g | 9.03 ± 0.29h | 5.62 ± 0.01f | 1.96 ± 0.01h | ND | 0.42 ± 0.01j | ND | 11.25 ± 0.22f | ND | |
| S3 | RG−U | 12.50 ± 0.85j | 4.79 ± 0.12i | 16.00 ± 0.93e | 4.55 ± 0.05h | 2.36 ± 0.00f | 0.24 ± 0.02d | 3.99 ± 0.19a | 0.52 ± 0.03d | 23.93 ± 1.08e | 1.42 ± 0.06f |
| RG−T | 14.54 ± 0.01i | 4.72 ± 0.05i | 16.65 ± 0.09e | 4.52 ± 0.05h | 2.27 ± 0.01g | ND | 0.71 ± 0.04h | ND | 3.35 ± 0.17k | ND | |
| XY−U | 37.24 ± 0.51c | 8.97 ± 0.19e | 18.99 ± 0.54c | 4.72 ± 0.03g | 1.80 ± 0.02j | 0.86 ± 0.02a | 3.99 ± 0.10a | 1.57 ± 0.03b | 35.63 ± 0.86a | 4.74 ± 0.09b | |
| XY−T | 15.20 ± 0.46i | 4.63 ± 0.15i | 8.59 ± 0.67hi | 4.44 ± 0.01hi | 1.89 ± 0.01i | 0.20 ± 0.01e | 0.82 ± 0.03d | 0.47 ± 0.01e | 8.13 ± 0.21g | 1.81 ± 0.04e | |
| S4 | RG−U | 5.09 ± 0.15lm | 2.17 ± 0.04l | 6.33 ± 0.09j | 3.28 ± 0.02k | 1.19 ± 0.03n | 0.41 ± 0.00c | 3.25 ± 0.06c | 0.88 ± 0.02c | 31.03 ± 0.56b | 2.46 ± 0.03d |
| RG−T | 5.90 ± 0.03l | 2.49 ± 0.03k | 8.40 ± 0.05hi | 4.75 ± 0.05g | 2.30 ± 0.14g | ND | 0.65 ± 0.01h | ND | 7.18 ± 0.18h | 0.80 ± 0.02h | |
| XY−U | 23.71 ± 0.27c | 3.11 ± 0.03j | 10.40 ± 0.05g | 4.32 ± 0.11i | 1.38 ± 0.03m | 0.79 ± 0.02b | 2.66 ± 0.05d | 1.55 ± 0.03b | 30.23 ± 0.55c | 4.49 ± 0.03c | |
| XY−T | 8.28 ± 0.59k | 2.65 ± 0.03k | 4.83 ± 0.05k | 4.84 ± 0.02g | 1.73 ± 0.01k | ND | 0.38 ± 0.01j | ND | 3.90 ± 0.05j | 0.50 ± 0.00i | |
| S5 | RG−U | 4.32 ± 0.33m | 1.74 ± 0.10m | 5.39 ± 0.20jk | 2.36 ± 0.16mn | 0.76 ± 0.04o | 0.13 ± 0.00f | 3.54 ± 0.10b | 0.32 ± 0.00f | 25.26 ± 0.61d | 0.86 ± 0.01g |
| RG−T | 2.81 ± 0.23n | 1.80 ± 0.08m | 7.59 ± 0.43i | 2.25 ± 0.01n | 1.54 ± 0.00l | ND | 0.16 ± 0.00k | ND | 6.84 ± 0.24h | 0.34 ± 0.01j | |
| XY−U | 8.74 ± 0.12k | 2.11 ± 0.05l | 5.72 ± 0.10jk | 2.43 ± 0.01m | 0.67 ± 0.04p | 0.79 ± 0.03b | 1.74 ± 0.02e | 1.87 ± 0.04a | 29.74 ± 0.66c | 6.24 ± 0.10a | |
| XY−T | 8.11 ± 0.36k | 1.85 ± 0.04m | 5.59 ± 0.22jk | 2.73 ± 0.00l | 0.69 ± 0.01p | ND | 0.03 ± 0.00l | ND | 2.31 ± 0.05l | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, L.; Xie, H.; Xiang, N.; Wang, M.; Han, S.; Pan, M.; Guo, X.; Zhang, W. Dynamic Changes in Anthocyanin Accumulation and Cellular Antioxidant Activities in Two Varieties of Grape Berries during Fruit Maturation under Different Climates. Molecules 2022, 27, 384. https://doi.org/10.3390/molecules27020384
Qin L, Xie H, Xiang N, Wang M, Han S, Pan M, Guo X, Zhang W. Dynamic Changes in Anthocyanin Accumulation and Cellular Antioxidant Activities in Two Varieties of Grape Berries during Fruit Maturation under Different Climates. Molecules. 2022; 27(2):384. https://doi.org/10.3390/molecules27020384
Chicago/Turabian StyleQin, Liuwei, Hui Xie, Nan Xiang, Min Wang, Shouan Han, Mingqi Pan, Xinbo Guo, and Wen Zhang. 2022. "Dynamic Changes in Anthocyanin Accumulation and Cellular Antioxidant Activities in Two Varieties of Grape Berries during Fruit Maturation under Different Climates" Molecules 27, no. 2: 384. https://doi.org/10.3390/molecules27020384
APA StyleQin, L., Xie, H., Xiang, N., Wang, M., Han, S., Pan, M., Guo, X., & Zhang, W. (2022). Dynamic Changes in Anthocyanin Accumulation and Cellular Antioxidant Activities in Two Varieties of Grape Berries during Fruit Maturation under Different Climates. Molecules, 27(2), 384. https://doi.org/10.3390/molecules27020384