Revealing the Inner Changes of Component Composition Derived from DOM PARAFAC Based on Two-Dimensional Correlation Spectroscopy
Abstract
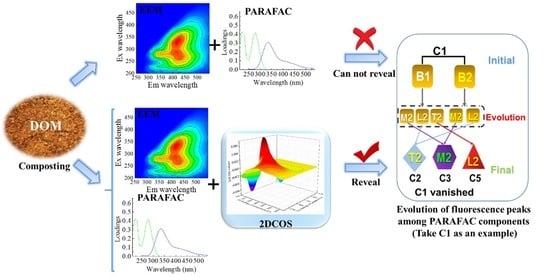
:1. Introduction
2. Results and Discussion
2.1. DOM Characterization Using PARAFAC Analysis
2.2. Distribution of PARAFAC Molecules during Composting Process
2.3. Characterization of the Composting-Derived DOM Dynamics Using the Excitation Loadings of PARAFAC Molecules Coupled with 2DCOS
2.4. Characterization of the Composting-Derived DOM Dynamics Using the Excitation Spectra of PARAFAC Molecules Coupled with Hetero-2DCOS
2.5. Change Speed of the Fluorescence Peaks in Different Composting Materials
2.6. Dynamics of DOM Molecules during Composting
3. Experimental Section
3.1. Composting Procedure
3.2. Extraction of DOM
3.3. Fluorescence Spectroscopy
3.4. Parallel Factor Analysis
3.5. Two-Dimensional Correlation Spectra Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, X.-S.; Xi, B.-D.; Zhang, Z.-Y.; Gao, R.-T.; Tan, W.-B.; Cui, D.-Y.; Yuan, Y. Composition, removal, redox, and metal complexation properties of dissolved organic nitrogen in composting leachates. J. Hazard. Mater. 2015, 283, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Huang, G.; Huang, J.; Zeng, J.; Han, L. Mechanism and kinetics of organic matter degradation based on particle structure variation during pig manure aerobic composting. J. Hazard. Mater. 2015, 292, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Said-Pullicino, D.; Kaiser, K.; Guggenberger, G.; Gigliotti, G. Changes in the chemical composition of water-extractable organic matter during composting: Distribution between stable and labile organic matter pools. Chemosphere 2007, 66, 2166–2176. [Google Scholar] [CrossRef] [PubMed]
- He, X.-S.; Xi, B.-D.; Li, W.-T.; Gao, R.-T.; Zhang, H.; Tan, W.-B.; Huang, C.-H. Insight into the composition and evolution of compost-derived dissolved organic matter using high-performance liquid chromatography combined with Fourier transform infrared and nuclear magnetic resonance spectra. J. Chromatogr. A 2015, 1420, 83–91. [Google Scholar] [CrossRef]
- Cui, H.-Y.; Zhao, Y.; Chen, Y.-N.; Zhang, X.; Wang, X.-Q.; Lu, Q.; Jia, L.-M.; Wei, Z.-M. Assessment of phytotoxicity grade during composting based on EEM/PARAFAC combined with projection pursuit regression. J. Hazard. Mater. 2017, 326, 10–17. [Google Scholar] [CrossRef]
- He, X.; Xi, B.; Wei, Z.; Guo, X.; Li, M.; An, D.; Liu, H. Spectroscopic characterization of water extractable organic matter during composting of municipal solid waste. Chemosphere 2011, 82, 541–548. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S.; Bro, R. Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy. Mar. Chem. 2003, 82, 239–254. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence excitation− emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Aftab, B.; Hur, J. Unraveling complex removal behavior of landfill leachate upon the treatments of Fenton oxidation and MIEX® via two-dimensional correlation size exclusion chromatography (2D-CoSEC). J. Hazard. Mater. 2019, 362, 36–44. [Google Scholar] [CrossRef]
- Lv, B.; Xing, M.; Zhao, C.; Yang, J.; Xiang, L. Towards understanding the stabilization process in vermicomposting using PARAFAC analysis of fluorescence spectra. Chemosphere 2014, 117, 216–222. [Google Scholar] [CrossRef]
- Cui, H.; Wen, X.; Wu, Z.; Zhao, Y.; Lu, Q.; Wei, Z. Insight into complexation of Cd (II) and Cu (II) to fulvic acid based on feature recognition of PARAFAC combined with 2DCOS. J. Hazard. Mater. 2022, 440, 129758. [Google Scholar] [CrossRef]
- He, X.-S.; Xi, B.-D.; Gao, R.-T.; Zhang, H.; Dang, Q.-L.; Li, D.; Huang, C.-H. Insight into the composition and degradation potential of dissolved organic matter with different hydrophobicity in landfill leachates. Chemosphere 2016, 144, 75–80. [Google Scholar] [CrossRef]
- Yu, G.-H.; Luo, Y.-H.; Wu, M.-J.; Tang, Z.; Liu, D.-Y.; Yang, X.-M.; Shen, Q.-R. PARAFAC modeling of fluorescence excitation− emission spectra for rapid assessment of compost maturity. Bioresour. Technol. 2010, 101, 8244–8251. [Google Scholar] [CrossRef]
- Wu, C.; Li, Y.; Li, W.; Wang, K. Characterizing the distribution of organic matter during composting of sewage sludge using a chemical and spectroscopic approach. RSC Adv. 2015, 5, 95960–95966. [Google Scholar] [CrossRef]
- Noda, I.; Ozaki, Y. Two-Dimensional Correlation Spectroscopy: Applications in Vibrational and Optical Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Ryu, S.R.; Bae, W.M.; Hong, W.J.; Ihn, K.J.; Jung, Y.M. Characterization of chain transfer reaction during radical polymerization of silver nanocomposite polyvinylpyrrolidone by using 2D hetero-spectral IR/NMR correlation spectroscopy. Vib. Spectrosc. 2012, 60, 168–172. [Google Scholar] [CrossRef]
- Chen, W.; Habibul, N.; Liu, X.-Y.; Sheng, G.-P.; Yu, H.-Q. FTIR and synchronous fluorescence heterospectral two-dimensional correlation analyses on the binding characteristics of copper onto dissolved organic matter. Environ. Sci. Technol. 2015, 49, 2052–2058. [Google Scholar] [CrossRef]
- Yuan, D.-h.; Guo, X.-j.; Wen, L.; He, L.-s.; Wang, J.-g.; Li, J.-q. Detection of Copper (II) and Cadmium (II) binding to dissolved organic matter from macrophyte decomposition by fluorescence excitation-emission matrix spectra combined with parallel factor analysis. Environ. Pollut. 2015, 204, 152–160. [Google Scholar] [CrossRef]
- Zhang, X.; Marcé, R.; Armengol, J.; Tauler, J. Distribution of dissolved organic matter in freshwaters using excitation emission fluorescence and Multivariate Curve Resolution. Chemosphere 2014, 111, 120–128. [Google Scholar] [CrossRef]
- Tedetti, M.; Cuet, P.; Guigue, C.; Goutx, M. Characterization of dissolved organic matter in a coral reef ecosystem subjected to anthropogenic pressures (La Réunion Island, Indian Ocean) using multi-dimensional fluorescence spectroscopy. Sci. Total Environ. 2011, 409, 2198–2210. [Google Scholar] [CrossRef]
- Coble, P.G. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar. Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Waite, T.D.; Ruiz, G.M. Distinguishing between terrestrial and autochthonous organic matter sources in marine environments using fluorescence spectroscopy. Mar. Chem. 2008, 108, 40–58. [Google Scholar] [CrossRef]
- Guo, W.; Xu, J.; Wang, J.; Wen, Y.; Zhuo, J.; Yan, Y. Characterization of dissolved organic matter in urban sewage using excitation emission matrix fluorescence spectroscopy and parallel factor analysis. J. Environ. Sci. 2010, 22, 1728–1734. [Google Scholar] [CrossRef] [Green Version]
- Stedmon, C.A.; Markager, S. Resolving the variability in dissolved organic matter fluorescence in a temperate estuary and its catchment using PARAFAC analysis. Limnol. Oceanogr. 2005, 50, 686–697. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, J.; Meng, F. Interactions between protein-like and humic-like components in dissolved organic matter revealed by fluorescence quenching. Water Res. 2015, 68, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Stedmon, C.A.; Markager, S. Tracing the production and degradation of autochthonous fractions of dissolved organic matter by fluorescence analysis. Limnol. Oceanogr. 2005, 50, 1415–1426. [Google Scholar] [CrossRef]
- Cory, R.M.; McKnight, D.M. Fluorescence spectroscopy reveals ubiquitous presence of oxidized and reduced quinones in dissolved organic matter. Environ. Sci. Technol. 2005, 39, 8142–8149. [Google Scholar] [CrossRef]
- Cui, H.; Shi, J.; Qiu, L.; Zhao, Y.; Wei, Z.; Wang, X.; Jia, L.; Li, J. Characterization of chromophoric dissolved organic matter and relationships among PARAFAC components and water quality parameters in Heilongjiang, China. Environ. Sci. Pollut. Res. 2016, 23, 10058–10071. [Google Scholar] [CrossRef]
- Holbrook, R.D.; Yen, J.H.; Grizzard, T.J. Characterizing natural organic material from the Occoquan Watershed (Northern Virginia, US) using fluorescence spectroscopy and PARAFAC. Sci. Total Environ. 2006, 361, 249–266. [Google Scholar] [CrossRef]
- He, X.-S.; Xi, B.-D.; Li, D.; Guo, X.-J.; Cui, D.-Y.; Pan, H.-W.; Ma, Y. Influence of the composition and removal characteristics of organic matter on heavy metal distribution in compost leachates. Environ. Sci. Pollut. Res. 2014, 21, 7522–7529. [Google Scholar] [CrossRef]
- Wang, K.; He, C.; You, S.; Liu, W.; Wang, W.; Zhang, R.; Qi, H.; Ren, N. Transformation of organic matters in animal wastes during composting. J. Hazard. Mater. 2015, 300, 745–753. [Google Scholar] [CrossRef]
- Murphy, K.R.; Hambly, A.; Singh, S.; Henderson, R.K.; Baker, A.; Stuetz, R.; Khan, S.J. Organic matter fluorescence in municipal water recycling schemes: Toward a unified PARAFAC model. Environ. Sci. Technol. 2011, 45, 2909–2916. [Google Scholar] [CrossRef]
- He, X.-S.; Xi, B.-D.; Li, X.; Pan, H.-W.; An, D.; Bai, S.-G.; Li, D.; Cui, D.-Y. Fluorescence excitation–emission matrix spectra coupled with parallel factor and regional integration analysis to characterize organic matter humification. Chemosphere 2013, 93, 2208–2215. [Google Scholar] [CrossRef]
- Marhuenda-Egea, F.; Martínez-Sabater, E.; Jordá, J.; Moral, R.; Bustamante, M.; Paredes, C.; Pérez-Murcia, M. Dissolved organic matter fractions formed during composting of winery and distillery residues: Evaluation of the process by fluorescence excitation–emission matrix. Chemosphere 2007, 68, 301–309. [Google Scholar] [CrossRef]
- Wei, Z.; Zhao, X.; Zhu, C.; Xi, B.; Zhao, Y.; Yu, X. Assessment of humification degree of dissolved organic matter from different composts using fluorescence spectroscopy technology. Chemosphere 2014, 95, 261–267. [Google Scholar] [CrossRef]
- Hur, J.; Park, M.-H.; Schlautman, M.A. Microbial transformation of dissolved leaf litter organic matter and its effects on selected organic matter operational descriptors. Environ. Sci. Technol. 2009, 43, 2315–2321. [Google Scholar] [CrossRef]
- He, X.-S.; Xi, B.-D.; Pan, H.-W.; Li, X.; Li, D.; Cui, D.-Y.; Tang, W.-B.; Yuan, Y. Characterizing the heavy metal-complexing potential of fluorescent water-extractable organic matter from composted municipal solid wastes using fluorescence excitation–emission matrix spectra coupled with parallel factor analysis. Environ. Sci. Pollut. Res. 2014, 21, 7973–7984. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Bahram, M.; Bro, R.; Stedmon, C.; Afkhami, A. Handling of Rayleigh and Raman scatter for PARAFAC modeling of fluorescence data using interpolation. J. Chemom. A J. Chemom. Soc. 2006, 20, 99–105. [Google Scholar] [CrossRef]






| Fluorescent Components | Peak Position λEx/Em (nm) | Description and Probable Source | Reference λEx/Em (nm) |
|---|---|---|---|
| C1 | 220, 270/305 | Tyrosine-like compounds characterized as fluorescence peak B1(C1) and peak B2(C1) | Peak B1 = 220–235/304–310 [20] Peak B2 = 270–280/304–310 [20] Component 4 = 274/306 [7] Component 4 = 260–290/290–340 [11] |
| C2 | 230, 280/340 | Tryptophan-like compounds characterized as fluorescence peak T1(C2) and peak T2(C2) | Peak T1 = 220–235/334–360 [21] Peak T2 = 270–280/334–360 [21] Component 7 = 240, 300/338 [22] Component 3 = 220, 280/340 [11] |
| C3 | 245, 290, 320–360/410 | Humic-like compounds characterized as fluorescence peak A1(C3), peak M1(C3), and peak M2(C3) | Peak A = 260/380–460; Peak M = 312/420–480 [21] Component 1 = 230, 330/410 [23] Component 8 = 250, 380/416 [22] Component 2 = <250, 305/412 [24] |
| C4 | 270, 370/460 | A combination of humic-like peak A2(C4) and the ubiquitous humic-like peak C(C4) | Peak A = 260/380–460; Peak C = 350/420–480 [21] Component 3 = 270, 360/478 [7] Component 1 = 250–275(280–400)/370–500 nm [25] Component 2 = 240, 360/466 [11] |
| C5 | 220, 280, 330/405 | Terrestrial humic-like substances; Biological; similar to the lawsone plotted in fluorescence spectra (peak L1(C5), peak L2(C5), and peak L2(C5) shoulder). | Component 3 = 295/398 [26] Component 2 = 315/418 [22] Component Q3 = 250–260/388 nm [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, H.; Xie, L.; Zhang, G.; Zhao, Y.; Wei, Z. Revealing the Inner Changes of Component Composition Derived from DOM PARAFAC Based on Two-Dimensional Correlation Spectroscopy. Molecules 2022, 27, 7316. https://doi.org/10.3390/molecules27217316
Cui H, Xie L, Zhang G, Zhao Y, Wei Z. Revealing the Inner Changes of Component Composition Derived from DOM PARAFAC Based on Two-Dimensional Correlation Spectroscopy. Molecules. 2022; 27(21):7316. https://doi.org/10.3390/molecules27217316
Chicago/Turabian StyleCui, Hongyang, Lina Xie, Guogang Zhang, Yue Zhao, and Zimin Wei. 2022. "Revealing the Inner Changes of Component Composition Derived from DOM PARAFAC Based on Two-Dimensional Correlation Spectroscopy" Molecules 27, no. 21: 7316. https://doi.org/10.3390/molecules27217316
APA StyleCui, H., Xie, L., Zhang, G., Zhao, Y., & Wei, Z. (2022). Revealing the Inner Changes of Component Composition Derived from DOM PARAFAC Based on Two-Dimensional Correlation Spectroscopy. Molecules, 27(21), 7316. https://doi.org/10.3390/molecules27217316