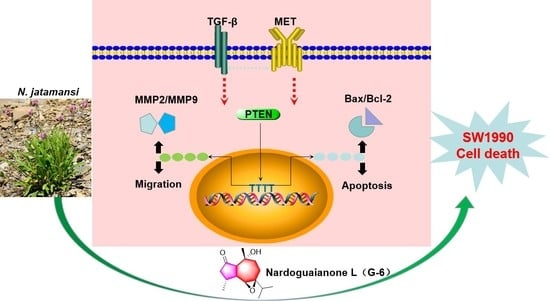
Potential Anti-Tumor Activity of Nardoguaianone L Isolated from Nardostachys jatamansi DC. in SW1990 Cells
Abstract
:1. Introduction
2. Results
2.1. G-6 Inhibited SW1990 Cells Proliferation and Colony Formation
2.2. G-6 Induced Cell Apoptosis in SW1990 Cells
2.3. Effects of G-6 on In Vitro SW1990 Cells Migration
2.4. Quantitative Analysis of Protein
2.5. Analysis for Differentially Expressed Proteins (DEPs)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Immunofluorescence Analysis
4.5. Morphological Analysis
4.6. Apoptosis Assay
4.7. Scratch-Induced Migration Assay
4.8. Protein Extraction and Digest
4.9. Peptides Labeling and MS Analysis
4.10. RPLC Analysis
4.11. UPLC-MS/MS Analysis
4.12. Protein Identification and Quantification
4.13. Bioinformatics
4.14. Data Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yue, J.; Li, W.; Wang, Y. Superiority verification of deep learning in the identification of medicinal plants: Taking Paris polyphylla var. yunnanensis as an example. Front. Plant Sci. 2021, 12, 752863. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xu, M.; Gao, Y.; Deng, Z.; Cao, H.; Zhang, W.; Wang, Q.; Zhang, B.; Song, G.; Zhan, Y.; et al. Matrine induces caspase-independent program cell death in hepatocellular carcinoma through bid-mediated nuclear translocation of apoptosis inducing factor. Mol. Cancer 2014, 13, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Li, X.; Zhang, J.; He, T.; Huang, J.; Zhang, Z.; Wang, Y.; Hafeez, M.; Zhou, S.; Ren, X.; et al. Comprehensive metabolome and volatilome analyses in eggplant and tomato reveal their differential responses to tuta absoluta infestation. Front. Plant Sci. 2021, 12, 757230. [Google Scholar] [CrossRef] [PubMed]
- Galanina, N.; Goodman, A.M.; Cohen, P.R.; Frampton, G.M.; Kurzrock, R. Successful treatment of HIV-associated kaposi sarcoma with immune checkpoint blockade. Cancer Immunol. Res. 2018, 6, 1129–1135. [Google Scholar] [CrossRef] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Wang, S.; Shi, J.; Sun, Z.; Lei, Z.; Yin, Z.; Qian, Z.; Tang, H.; Xie, H. Genotypic and environmental effects on the volatile chemotype of Valeriana jatamansi jones. Front. Plant Sci. 2018, 9, 1003. [Google Scholar] [CrossRef]
- Li, R.; Wang, Z.M.; Wang, Y.; Dong, X.Q.; Zhang, L.H.; Wang, T.; Zhu, Y.; Gao, X.M.; Wu, H.H.; Xu, Y.T. Antidepressant activities and regulative effects on serotonin transporter of Nardostachys jatamansi DC. J. Ethnopharmacol. 2020, 268, 113601. [Google Scholar] [CrossRef]
- Chaudhary, S.; Chandrashekar, K.S.; Pai, K.S.; Setty, M.M.; Devkar, R.A.; Reddy, N.D.; Shoja, M.H. Evaluation of antioxidant and anticancer activity of extract and fractions of Nardostachys jatamansi DC. in breast carcinoma. BMC Complement. Altern. Med. 2015, 15, 50. [Google Scholar] [CrossRef] [Green Version]
- Suryavanshi, S.; Raina, P.; Deshpande, R.; Kaul, G.R. Nardostachys jatamansi root extract modulates the growth of IMR-32 and SK-N-MC neuroblastoma cell lines through MYCN mediated regulation of MDM2 and p53. Pharmacogn. Magn. 2017, 13, 21–24. [Google Scholar] [CrossRef]
- Anupama, K.P.; Shilpa, O.; Antony, A.; Gurushankara, H.P. Jatamansinol from Nardostachys jatamansi: A multi-targeted neuroprotective agent for Alzheimer’s disease. J. Biomol. Struct. Dyn. 2021, 2, 1–21. [Google Scholar] [CrossRef]
- Bian, L.H.; Wao, Y.Z.; Zhao, C.B.; Li, Q.Y.; Shi, J.L.; Guo, J.Y. Nardosinone alleviates parkinson’s disease symptoms in mice by regulating dopamine D2 receptor. Evid. Based Complement. Altern. Med. 2021, 13, 6686965. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Khan, M.A.; Kamal, Y.T.; Ahmad, J.; Fahmy, U.A.; Kotta, S.; Alhakamy, N.A.; Ahmad, S. Effect of Nardostachys jatamansi DC. on apoptosis, inflammation and oxidative stress induced by doxorubicin in Wistar Rats. Plants 2020, 9, 1579. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.M.; Wang, K.; Meng, X.H.; Zheng, Y.D.; Wang, C.B.; Chai, T.; Naghavi, M.R.; Sang, C.Y.; Yang, J.L. Terpenoids from Nardostachys jatamansi DC. and their inhibition on human pancreatic cancer cell lines. Phytochemistry 2022, 200, 113228. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.D.; Ma, L.M.; Lu, J.J.; Chai, T.; Naghavi, M.R.; Ma, J.Y.; Sang, C.Y.; Yang, J.L. Nardoguaianone L Isolated from Nardostachys jatamansi Improved the Effect of Gemcitabine Chemotherapy via Regulating AGE Signaling Pathway in SW1990 Cells. Molecules 2022, 27, 6849. [Google Scholar] [CrossRef]
- Ma, Y.; Kuang, Y.; Bo, W.; Liang, Q.; Zhu, W.; Cai, M.; Tian, Z. Exercise training alleviates cardiac fibrosis through increasing fibroblast growth factor 21 and regulating TGF-β1-Smad2/3-MMP2/9 signaling in mice with myocardial infarction. Int. J. Mol. Sci. 2021, 22, 12341. [Google Scholar] [CrossRef]
- Xiao, S.; Shun, X.; Yu, R.R.; Ai, N.; Fan, X.H. Rapid screening natural-origin lipase inhibitors from hypolipidemic decoctions by ultrafiltration combined with liquid chromatography-mass spectrometry. J. Pharmaceut. Biomed. 2015, 104, 67–74. [Google Scholar] [CrossRef]
- Zhang, L.; Qiang, P.; Yu, J.; Miao, Y.; Chen, Z.; Qu, J.; Zhao, Q.; Chen, Z.; Liu, Y.; Yao, X.; et al. Identification of compound CA-5f as a novel late-stage autophagy inhibitor with potent anti-tumor effect against non-small cell lung cancer. Autophagy 2019, 15, 391–406. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.L.; Guo, M.Q. Screening for natural inhibitors of topoisomerases I from Rhamnus davurica by affinity ultrafiltration and high-performance liquid chromatography-mass spectrometry. Front. Plant Sci. 2017, 8, 1521. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Feng, W.; Shi, J.; Chen, L.; Huang, J.; Lin, T. circRIP2 accelerates bladder cancer progression via miR-1305/Tgf-β2/smad3 pathway. Mol. Cancer 2020, 19, 23. [Google Scholar] [CrossRef] [Green Version]
- Cui, D.; Dai, J.; Keller, J.M.; Mizokami, A.; Xia, S.; Keller, E. Notch pathway inhibition using PF-03084014, a γ-secretase inhibitor (GSI), enhances the antitumor effect of docetaxel in prostate cancer. Clin. Cancer Res. 2015, 21, 4619–4629. [Google Scholar] [CrossRef]
- Duan, L.; Dong, S.; Huang, K.; Cong, Y.; Luo, S.; Zhang, J.Z.H. Computational analysis of binding free energies, hotspots and the binding mechanism of Bcl-xL/Bcl-2 binding to Bad/Bax. Phys. Chem. Chem. Phys. 2021, 23, 2025–2037. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhang, L. EGR1/2 inhibits papillary thyroid carcinoma cell growth by suppressing the expression of PTEN and BAX. Biochem. Genet. 2021, 59, 1544–1557. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Matthews, J.; Parvin, S.; Sarosiek, K.A.; Zhao, D.; Jiang, X.; Isik, E.; Letai, A.; Lossos, I.S. Direct and immune-mediated cytotoxicity of interleukin-21 contributes to antitumor effects in mantle cell lymphoma. Blood 2015, 126, 1555–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.X.; Feng, L.H.; Hao, J.; Zhang, Y.B.; Sheng, Z. Different proteome profiles between male and female populus cathayana exposed to UV-B radiation. Front. Plant Sci. 2017, 8, 320. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.Y.; Hu, M.; Dai, M.H.; Xiong, J.; Zhang, S.; Wu, H.J.; Zhang, S.S.; Gong, Z.J. Upregulation of the long non-coding RNA AFAP1-AS1 affects the proliferation, invasion and survival of tongue squamous cell carcinoma via the Wnt/β-catenin signaling pathway. Mol. Cancer 2018, 17, 3. [Google Scholar] [CrossRef] [Green Version]
- Gélinas, R.; Mailleux, F.; Dontaine, J.; Bultot, L.; Demeulder, B.; Ginion, A.; Daskalopoulos, E.P.; Esfahani, H.; Dubois-Deruy, E.; Lauzier, B.; et al. AMPK activation counteracts cardiac hypertrophy by reducing O-GlcNAcylation. Nat. Commun. 2018, 9, 374. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Wang, C.; Yan, Y.; Jia, H.; Guo, X. Overexpression of cotton GhMPK11 decreases disease resistance through the gibberellin signaling pathway in transgenic nicotiana benthamiana. Front. Plant Sci. 2016, 7, 689. [Google Scholar] [CrossRef] [Green Version]
- Choi, G.E.; Lee, H.J.; Chae, C.W.; Cho, J.H.; Jung, Y.H.; Kim, J.S.; Kim, S.Y.; Lim, J.R.; Han, H.J. BNIP3L/NIX-mediated mitophagy protects against glucocorticoid-induced synapse defects. Nat. Commun. 2021, 12, 487. [Google Scholar] [CrossRef]
- Laurent, V.; Guérard, A.; Mazerolles, C.; Le Gonidec, S.; Toulet, A.; Nieto, L.; Zaidi, F.; Majed, B.; Garandeau, D.; Socrier, Y.; et al. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat. Commun. 2016, 7, 10230. [Google Scholar] [CrossRef] [Green Version]
- Griss, J.; Vinterhalter, G.; Schwämmle, V.I. IsoProt: A complete and reproducible workflow to analyze iTRAQ/TMT experiments. J. Proteome Res. 2019, 18, 1751–1759. [Google Scholar] [CrossRef]
- Burke, J.E.; Longhurst, A.D.; Merkurjev, D.; Sales-Lee, J.; Rao, B.; Moresco, J.J.; Yates, J.R.; Li, J.J.; Madhani, H.D. Spliceosome profiling visualizes operations of a dynamic RNP at nucleotide resolution. Cell 2018, 173, 1014–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moser, E.K.; Roof, J.; Dybas, J.M.; Spruce, L.A.; Seeholzer, S.H.; Cancro, M.P.; Oliver, P.M. The E3 ubiquitin ligase Itch restricts antigen-driven B cell responses. J. Exp. Med. 2019, 216, 2170–2183. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sang, C.-Y.; Zheng, Y.-D.; Ma, L.-M.; Wang, K.; Wang, C.-B.; Chai, T.; Eshbakova, K.A.; Yang, J.-L. Potential Anti-Tumor Activity of Nardoguaianone L Isolated from Nardostachys jatamansi DC. in SW1990 Cells. Molecules 2022, 27, 7490. https://doi.org/10.3390/molecules27217490
Sang C-Y, Zheng Y-D, Ma L-M, Wang K, Wang C-B, Chai T, Eshbakova KA, Yang J-L. Potential Anti-Tumor Activity of Nardoguaianone L Isolated from Nardostachys jatamansi DC. in SW1990 Cells. Molecules. 2022; 27(21):7490. https://doi.org/10.3390/molecules27217490
Chicago/Turabian StyleSang, Chun-Yan, Yi-Dan Zheng, Li-Mei Ma, Kai Wang, Cheng-Bo Wang, Tian Chai, Komila A. Eshbakova, and Jun-Li Yang. 2022. "Potential Anti-Tumor Activity of Nardoguaianone L Isolated from Nardostachys jatamansi DC. in SW1990 Cells" Molecules 27, no. 21: 7490. https://doi.org/10.3390/molecules27217490
APA StyleSang, C. -Y., Zheng, Y. -D., Ma, L. -M., Wang, K., Wang, C. -B., Chai, T., Eshbakova, K. A., & Yang, J. -L. (2022). Potential Anti-Tumor Activity of Nardoguaianone L Isolated from Nardostachys jatamansi DC. in SW1990 Cells. Molecules, 27(21), 7490. https://doi.org/10.3390/molecules27217490