A Two-Stage Culture Strategy for Scenedesmus sp. FSP3 for CO2 Fixation and the Simultaneous Production of Lutein under Light and Salt Stress
Abstract
:1. Introduction
2. Results
2.1. Growth and CO2 Fixation Rates of Microalgae under Different CO2 Concentrations
2.2. Addition of CO2 Solubilization Enhancer for Better Carbon Absorption
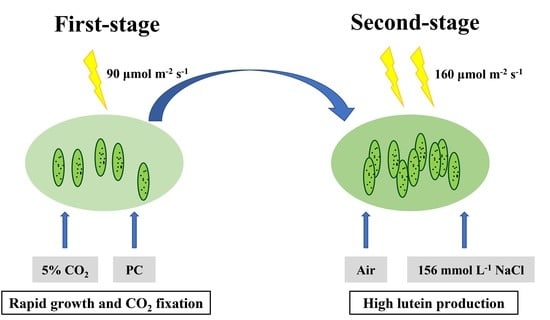
2.3. Two-Stage Strategy Promoting Lutein Accumulation under Light and Salt Stress
2.3.1. Effect of Light Intensity on Lutein Content
2.3.2. Effect of Salt Concentration on Lutein Content
2.4. Response Surface Methodology for Lutein Production
3. Discussion
4. Materials and Methods
4.1. Microalgae Species and Cultivation
4.2. Cultivate Model: Two-Stage Culture
4.3. Measurement of Cell Growth
4.4. Measurement of Carbon Dioxide Biofixation Rate
4.5. Extraction and Determination of Lutein
4.6. Response Surface Methodology for Lutein Yield
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- International Energy Agency-IEA. Global Energy Review: CO2 Emissions in 2021. 2022. Available online: https://www.iea.org/reports/global-energy-review-co2-emissions-in-2021-2 (accessed on 30 August 2022).
- Daneshvar, E.; Wicker, R.J.; Show, P.-L.; Bhatnagar, A. Biologically-mediated carbon capture and utilization by microalgae towards sustainable CO2 biofixation and biomass valorization—A review. Chem. Eng. J. 2021, 427, 130884. [Google Scholar] [CrossRef]
- Prasad, R.; Gupta, S.K.; Shabnam, N.; Oliveira, C.Y.B.; Nema, A.K.; Ansari, F.A.; Bux, F. Role of microalgae in global CO2 sequestration: Physiological mechanism, recent development, challenges, and future prospective. Sustainability 2021, 13, 13061. [Google Scholar] [CrossRef]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef] [PubMed]
- Jahns, P.; Holzwarth, A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta 2012, 1817, 182–193. [Google Scholar] [CrossRef] [Green Version]
- Saha, S.K.; Ermis, H.; Murray, P. Marine microalgae for potential lutein production. Appl. Sci. 2020, 10, 6457. [Google Scholar] [CrossRef]
- Llamas, B.; Suárez-Rodríguez, M.C.; González-López, C.V.; Mora, P.; Acién, F.G. Techno-economic analysis of microalgae related processes for CO2 bio-fixation. Algal Res. 2021, 57, 102339. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Y.; Li, S.; Nagarajan, D.; Varjani, S.; Lee, D.-J.; Chang, J.-S. Recent advances in lutein production from microalgae. Renew. Sustain. Energy Rev. 2021, 153, 111795. [Google Scholar] [CrossRef]
- Tang, D.; Han, W.; Li, P.; Miao, X.; Zhong, J. CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour. Technol. 2011, 102, 3071–3076. [Google Scholar] [CrossRef]
- Nayak, M.; Suh, W.I.; Lee, B.; Chang, Y.K. Enhanced carbon utilization efficiency and FAME production of Chlorella sp. HS2 through combined supplementation of bicarbonate and carbon dioxide. Energy Convers. Manag. 2018, 156, 45–52. [Google Scholar] [CrossRef]
- Zhu, Y.; Cheng, J.; Xu, X.; Lu, H.; Wang, Y.; Li, X.; Yang, W. Using polyethylene glycol to promote Nannochloropsis oceanica growth with 15 vol% CO2. Sci. Total Environ. 2020, 720, 137598. [Google Scholar] [CrossRef]
- Sun, Z.L.; Xin, M.R.; Li, P.; Sun, L.Q.; Wang, S.K. Enhancing CO2 utilization by a physical absorption-based technique in microalgae culture. Bioprocess Biosyst. Eng. 2021, 44, 1901–1912. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Sevilla, J.M.; Acien Fernandez, F.G.; Molina Grima, E. Biotechnological production of lutein and its applications. Appl. Microbiol. Biotechnol. 2010, 86, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Gong, S.; Chen, Z.; Xia, J.; Xiang, W. Potential microalgal strains for converting flue gas CO2 into biomass. J. Appl. Phycol. 2020, 33, 47–55. [Google Scholar] [CrossRef]
- Banerjee, A.; Guria, C.; Maiti, S.K.; Banerjee, C.; Shukla, P. Carbon bio-fixation, effect of physicochemical factors and carbon supply strategies by Nannochloropsis sp. using flue gas and fertilizer. Biomass Bioenergy 2019, 125, 95–104. [Google Scholar] [CrossRef]
- Ma, S.; Li, D.; Yu, Y.; Li, D.; Yadav, R.S.; Feng, Y. Application of a microalga, Scenedesmus obliquus PF3, for the biological removal of nitric oxide (NO) and carbon dioxide. Environ. Pollut. 2019, 252 Pt A, 344–351. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chen, C.-Y.; Yeh, K.-L.; Chen, W.-M.; Lin, C.-Y.; Chang, J.-S. Characterization of photosynthetic carbon dioxide fixation ability of indigenous Scenedesmus obliquus isolates. Biochem. Eng. J. 2010, 53, 57–62. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, W.; Wang, J.; Chen, Y.; Shen, S.; Liu, T. Utilization of simulated flue gas for cultivation of Scenedesmus dimorphus. Bioresour. Technol. 2013, 128, 359–364. [Google Scholar] [CrossRef]
- Choi, H.I.; Hwang, S.W.; Kim, J.; Park, B.; Jin, E.; Choi, I.G.; Sim, S.J. Augmented CO2 tolerance by expressing a single H(+)-pump enables microalgal valorization of industrial flue gas. Nat. Commun. 2021, 12, 6049. [Google Scholar] [CrossRef]
- Chinnasamy, S.; Ramakrishnan, B.; Bhatnagar, A.; Das, K.C. Biomass production potential of a wastewater alga Chlorella vulgaris ARC 1 under elevated levels of CO2 and temperature. Int. J. Mol. Sci. 2009, 10, 518–532. [Google Scholar] [CrossRef] [Green Version]
- Solovchenko, A.; Gorelova, O.; Selyakh, I.; Semenova, L.; Chivkunova, O.; Baulina, O.; Lobakova, E. Desmodesmus sp. 3Dp86E-1-a novel symbiotic chlorophyte capable of growth on pure CO2. Mar. Biotechnol. (NY) 2014, 16, 495–501. [Google Scholar] [CrossRef]
- Vaquero, I.; Mogedas, B.; Ruiz-Domínguez, M.C.; Vega, J.M.; Vílchez, C. Light-mediated lutein enrichment of an acid environment microalga. Algal. Res. 2014, 6, 70–77. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, Z.; Ho, S.-H.; Ruan, C.; Li, J.; Xie, Y.; Shi, X.; Liu, L.; Chen, J. Two-stage bioprocess for hyper-production of lutein from microalga Chlorella sorokiniana FZU60: Effects of temperature, light intensity, and operation strategies. Algal Res. 2014, 6, 70–77. [Google Scholar] [CrossRef]
- Rio-Chanona, E.A.D.; Ahmed, N.R.; Zhang, D.; Lu, Y.; Jing, K. Kinetic modeling and process analysis for Desmodesmus sp. lutein photo-production. AIChE J. 2017, 63, 2546–2554. [Google Scholar] [CrossRef]
- Schüler, L.M.; Santos, T.; Pereira, H.; Duarte, P.; Katkam, N.G.; Florindo, C.; Schulze, P.S.C.; Barreira, L.; Varela, J.C.S. Improved production of lutein and β-carotene by thermal and light intensity upshifts in the marine microalga Tetraselmis sp. CTP4. Algal Res. 2020, 45, 101732. [Google Scholar] [CrossRef]
- Xie, Y.; Ho, S.H.; Chen, C.N.; Chen, C.Y.; Ng, I.S.; Jing, K.J.; Chang, J.S.; Lu, Y. Phototrophic cultivation of a thermo-tolerant Desmodesmus sp. for lutein production: Effects of nitrate concentration, light intensity and fed-batch operation. Bioresour. Technol. 2013, 144, 435–444. [Google Scholar] [CrossRef]
- Alyabyev, A.; Andreyeva, I.; Rachimova, G. Influence of pH shift and salting on the energetics of microalgae Chlorella vulgaris and Dunaliella maritima. J. Therm. Anal. Calorim. 2011, 104, 201–207. [Google Scholar] [CrossRef]
- Martínez-Roldán, A.J.; Perales-Vela, H.V.; Cañizares-Villanueva, R.O.; Torzillo, G. Physiological response of Nannochloropsis sp. to saline stress in laboratory batch cultures. J. Appl. Phycol. 2013, 26, 115–121. [Google Scholar] [CrossRef]
- Ali, H.E.A.; El-fayoumy, E.A.; Rasmy, W.E.; Soliman, R.M.; Abdullah, M.A. Two-stage cultivation of Chlorella vulgaris using light and salt stress conditions for simultaneous production of lipid, carotenoids, and antioxidants. J. Appl. Phycol. 2020, 33, 227–239. [Google Scholar] [CrossRef]
- Bermejo, E.; Ruiz-Dominguez, M.C.; Cuaresma, M.; Vaquero, I.; Ramos-Merchante, A.; Vega, J.M.; Vilchez, C.; Garbayo, I. Production of lutein, and polyunsaturated fatty acids by the acidophilic eukaryotic microalga Coccomyxa onubensis under abiotic stress by salt or ultraviolet light. J. Biosci. Bioeng. 2018, 125, 669–675. [Google Scholar] [CrossRef]
- Cordero, B.F.; Obraztsova, I.; Couso, I.; Leon, R.; Vargas, M.A.; Rodriguez, H. Enhancement of lutein production in Chlorella sorokiniana (Chorophyta) by improvement of culture conditions and random mutagenesis. Mar. Drugs 2011, 9, 1607–1624. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Dash, S.K.; Sen, R. Process integration for microalgal lutein and biodiesel production with concomitant flue gas CO2 sequestration: A biorefinery model for healthcare, energy and environment. RSC Adv. 2015, 5, 73381–73394. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Karatza, D.; Chianese, S.; Iovine, A.; Casella, P.; Marino, T.; Musmarra, D. Bench-Scale Cultivation of Microalgae Scenedesmus almeriensis for CO2 Capture and Lutein Production. Energies 2019, 12, 2806. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Duan, D.; Sun, Y.; Han, Y.; Zhao, S. Enhancing Scenedesmus obliquus biofilm growth and CO2 fixation in a gas-permeable membrane photobioreactor integrated with additional rough surface. Algal Res. 2019, 43, 101620. [Google Scholar] [CrossRef]





| Microalgae Species | Gas Resource | Biomass Productivity (g L−1 d−1) | CO2 Fixation Rate (g L−1 d−1) | References |
|---|---|---|---|---|
| Scenedesmus obliquus PF3 | 10% CO2, 100 ppm NO | 0.40 | 0.75 | [16] |
| Chlorella sorokiniana GS03 | 5% CO2 | 0.35 | 0.66 | [14] |
| Heynigia riparia SX01 | 15% CO2 | 0.38 | 0.71 | [14] |
| Scenedesmus dimorphus | 15% CO2, 400 ppm SO2, 300 ppm NO | 0.35 | 0.66 | [18] |
| Scenedesmus obliquus AS-6-1 | 20% CO2 | 0.15 | 0.29 | [17] |
| Scenedesmus obliquus CNW-N | 20% CO2 | 0.21 | 0.39 | [17] |
| Nannochloropsis sp. | 11% CO2, 10% O2, 1–2% CO, 500 ppm CH4, N2 | 0.12 | 0.22 | [15] |
| Chlorella pyrenoidosa SJTU-2 | 10% CO2 | 0.14 | 0.26 | [9] |
| Scenedesmus obliquus SJTU-3 | 10% CO2 | 0.15 | 0.29 | [9] |
| Scenedesmus sp. FSP3 | 5% CO2 | 0.41 | 0.77 | This study |
| 10% CO2 | 0.41 | 0.77 | ||
| 15% CO2 | 0.35 | 0.66 | ||
| 20% CO2 | 0.28 | 0.53 |
| Microalgae | Lutein Productivity (mg L−1 d−1) | Lutein Content (mg g−1) | References |
|---|---|---|---|
| Chlorella sorokiniana | 0.84 | 3.00 | [31] |
| Coccomyxa onubensis | 0.55 | 6.20 | [22] |
| Chlorella minutissima | 0.67 | 6.37 | [32] |
| Scenedesmus almeriensis | 0.13 | 8.54 | [33] |
| Desmodesmus sp. F51 | 0.65 | 5.50 | [26] |
| Scenedesmus sp. FSP3 | 2.30 | 6.54 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhao, X.; Chang, J.-S.; Miao, X. A Two-Stage Culture Strategy for Scenedesmus sp. FSP3 for CO2 Fixation and the Simultaneous Production of Lutein under Light and Salt Stress. Molecules 2022, 27, 7497. https://doi.org/10.3390/molecules27217497
Li J, Zhao X, Chang J-S, Miao X. A Two-Stage Culture Strategy for Scenedesmus sp. FSP3 for CO2 Fixation and the Simultaneous Production of Lutein under Light and Salt Stress. Molecules. 2022; 27(21):7497. https://doi.org/10.3390/molecules27217497
Chicago/Turabian StyleLi, Jiawei, Xinqing Zhao, Jo-Shu Chang, and Xiaoling Miao. 2022. "A Two-Stage Culture Strategy for Scenedesmus sp. FSP3 for CO2 Fixation and the Simultaneous Production of Lutein under Light and Salt Stress" Molecules 27, no. 21: 7497. https://doi.org/10.3390/molecules27217497
APA StyleLi, J., Zhao, X., Chang, J. -S., & Miao, X. (2022). A Two-Stage Culture Strategy for Scenedesmus sp. FSP3 for CO2 Fixation and the Simultaneous Production of Lutein under Light and Salt Stress. Molecules, 27(21), 7497. https://doi.org/10.3390/molecules27217497