PMMA/ABS/CoCl2 Composites for Pharmaceutical Applications: Thermal, Antimicrobial, Antibiofilm, and Antioxidant Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Analysis by FT-IR
2.2. X-ray Diffraction
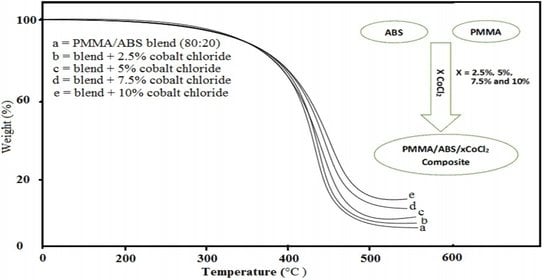
2.3. Thermal Analysis (TGA)
2.4. Mechanical Properties
2.5. Antibacterial Activity
2.6. Antibiofilm Activity
3. Antioxidant Activity
4. Materials and Method
4.1. Chemicals
4.2. Instruments
4.3. Preparation of PMMS/ABS/CoCl2 Composite Films
4.4. Antibacterial Methodology
4.5. Antibiofilm Assay
4.6. Antioxidant Assay
4.6.1. DPPH Free Radical Scavenging Activity
4.6.2. ABTH+ Radical Scavenging Activity
4.6.3. Ferric Reducing Antioxidant Power
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Shao, C.; Shang, P.; Mao, Y.; Li, Q.; Wu, C. Preparation and characterization of ABS/anhydrous cobalt chloride composites. Mater. Res. Express. 2018, 5, 015309–015324. [Google Scholar] [CrossRef]
- Mohamed, H.G.; Mohamed, A.A.; Waheedullah, G.; Naheed, S.; Mohammad, A.; Mohammad, J.; Othman, Y.A. Flexural thermal and dynamic mechanical properties of date palm fibres reinforced epoxy composites. J. Mater. Res. Technol. 2019, 8, 853–860. [Google Scholar] [CrossRef]
- Salih, H.Y. Effect of muti-walled carbon nanotubes on mechanical, thermal and rheological properties of poly propylene. J. Mater. Res. Technol. 2019, 8, 4725–4735. [Google Scholar] [CrossRef]
- Xie, H.; Cao, T.; Rodriguez-Lozano, F.J.; Luong-Van, E.K.; Rosa, V. Graphene for the development of the next-generation of bios for dental and medical applications. Dent. Mater. 2017, 33, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, E.; Saralidze, K.; Roth, A.K.; de Jong, J.J.A.; van den Bergh, J.P.W.; Lataster, A.; Brans, B.T.; Knetsch, M.L.W.; Djordjevic, I.; Willems, P.C.; et al. Synthesis and characterization of a new vertebroplasty cement based on gold-containing PMMA microspheres. Biomaterials 2016, 82, 60–70. [Google Scholar] [CrossRef]
- Naheed, S.; Othman, Y.A.; Zeyad, A.; Muhammad, J.; Waheedullah, G. Date palm reinforced epoxy composites: Tensile, impact and morphological properties. J. Mater. Res. Technol. 2019, 8, 3959–3969. [Google Scholar] [CrossRef]
- Penchal, R.M.; Ubaid, F.; Rana, A.S.; Adel, M.A.M. Comparative study of structural and mechanical properties of Al–Cu composites prepared by vacuum and microwave sintering techniques. J. Mater. Res. Technol. 2018, 7, 165–172. [Google Scholar] [CrossRef]
- Chang, L.; Zhang, X.; Shi, X.; Zhao, L.; Liu, X. Preparation and characterization of a novel antibacterial fiber modified by quaternary phosphonium salt on the surface of polyacrylonitrile fiber. Fibers Polym. 2014, 15, 2026–2031. [Google Scholar] [CrossRef]
- Xiaolong, G.; Yao, H.; Xiaoxiang, H.; Xiaojing, F.; Ying, L.; Hong, X.; Daming, W.; Chaoying, W. Mechanically enhanced electrical conductivity of polydimethylsiloxane-based composites by a hot embossing process. Polymers 2019, 11, 56. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.; Choi, W. Solid-phase photocatalytic degradation of PVC–TiO2 polymer composites. J. Photochem. Photobiol. A Chem. 2001, 143, 221–228. [Google Scholar] [CrossRef]
- Wei, D.; Ding, Y.; Wang, T.; Yang, J.; Guan, Y.; Zheng, A. Preparation of nonleaching antimicrobial polypropylene wax and its application in polypropylene. J. Appl. Polym. Sci. 2017, 134, 44190–44196. [Google Scholar] [CrossRef]
- Aimin, Z.; Gouqun, Z.; Yang, H.; Yanjin, G. Mechanical and thermal properties of ABS/PMMA/Potassium Titanate Whisker Composites. Polym.-Plast. Technol. Eng. 2017, 56, 382–390. [Google Scholar] [CrossRef]
- Weizhen, Z.; Jingwei, H.; Fang, L. Preparation and properties of antibacterial ABS plastics based on polymeric quaternary phosphonium salts antibacterial agents. Polym. Adv. Technol. 2019, 30, 2515–2522. [Google Scholar] [CrossRef]
- Sun, X.; Qian, Z.; Luo, L.; Yuan, Q.; Guo, X.; Tao, L.; Wei, Y.; Wang, X. Antibacterial adhesion of Poly(methyl methacrylate) modified by Borneol acrylate. ACS Appl. Mater. Interfaces 2016, 8, 28522–28528. [Google Scholar] [CrossRef] [PubMed]
- Fang-Lan, G.; Chen-Xi, G.; Hao-Bin, Z.; Zhi-Guo, J.; Zhong-Zhen, Y. Enhanced thermal conductivity and satisfactory flame retardancy of epoxy/alumina composites by combination with graphene Nano platelets and magnesium hydroxide. Compos. Part B 2016, 98, 134–140. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, S.; Han, J.W.; Abdal Dayem, A.; Eppakayala, V.; Kim, J.H. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomed. 2012, 7, 5901–5914. [Google Scholar] [CrossRef] [Green Version]
- Nagy, A.; Harrison, A.; Sabbani, S.; Munson, R.S.; Dutta, P.K.; Waldman, J. Silver nanoparticles embedded in zeolite membranes: Release of silver ions and mechanism of antibacterial action. Int. J. Nanomed. 2011, 6, 1833–1852. [Google Scholar] [CrossRef] [Green Version]
- Tawansi, A.; El-khodary, A.; Zidan, H.M.; Badr, S.I. The effect of MnCl2 filler on the optical window and the physical properties of PMMA films. Polym. Test. 2002, 21, 381–387. [Google Scholar] [CrossRef]
- Ravikumar, R.P.; Vasudev, R.T.; Bagavathi, R.S. A Pseudomonas guariconensis strain capable of promoting growth and controlling collar rot disease in Arachis hypogaea L. Plant Soil. 2015, 390, 369–381. [Google Scholar] [CrossRef]
- Elashmawi, I.S.; Elsayed, N.H.; Altalhi, F.A. The changes of spectroscopic, thermal and electrical properties of PVDF/PEO containing lithium nanoparticles. J. Alloys. Comp. 2014, 617, 877–883. [Google Scholar] [CrossRef]
- Ravikumar, R.P.; Disha, D.P.; Jaimika, B.; Parth, T.; Lindsay, R.T.; Vasudev, R.T. Induction of pre-chorismate, jasmonate and salicylate pathways by Burkholderia sp. RR18 in peanut seedlings. J. Appl. Microbiol. 2021, 131, 1417–1430. [Google Scholar] [CrossRef]
- Tekalign, K.; Vasudev, R.T.; Ravi, R.P. A novel strain of Pseudomonas inhibits Colletotrichum gloeosporioides and Fusarium oxysporum infections and promotes germination of Coffee. Rhizosphere 2017, 10, 9–15. [Google Scholar] [CrossRef]
- Qiao, W.; Wang, H.; Zhao, Y.; Han, Y. Mechanical properties of PVC/ABS composite material. Adv. Mater. Res. 2011, 217, 1170–1173. [Google Scholar] [CrossRef]
- Jayaseelan, C.; Rahuman, A.A.; Rajakumar, G.; Vishnu, K.A.; Santhosh, K.T.; Marimuthu, S.; Bagavan, A.; Kamaraj, C.; Zahir, A.A.; Elango, G. Synthesis of pediculocidal and larvicidal silver nanoparticles by leaf extract from heartleaf moonseed plant Tinospora cordifolia miers. Parasitol. Res. 2011, 109, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fiévet, F. Toxicological Impact Studies Based on Escherichia coli Bacteria in Ultrafine ZnO Nanoparticles Colloidal Medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef]
- Alghunaim, N.S. Spectroscopic analysis of PMMA/PVC blends containing CoCl2. Results Phys. 2015, 5, 331–336. [Google Scholar] [CrossRef] [Green Version]
- Balen, R.; da Costa, W.V.; de Lara Andrade, J.; Piai, J.F.; Muniz, E.C.; Companhoni, M.V.; Nakamura, T.U.; Lima, S.M.; da Cunha Andrade, L.H.; Bittencourt, P.R.S.; et al. Structural, thermal, optical properties and cytotoxicity of PMMA/ZnO fibers and films: Potential application in tissue engineering. Appl. Surf. Sci. 2016, 385, 257–267. [Google Scholar] [CrossRef]
- Annie, F.; Bernadette, G.; Philippe, H.L.; Isabelle, C. Evaluation of three techniques for detection of low-level methicillin-resistant Staphylococcus aureus (MRSA): A disk diffusion method with Cefoxitin and Moxalactam, the vitek 2 System, and the MRSA-Screen Latex agglutination test. J. Clin. Microbiol. 2002, 40, 2766–2771. [Google Scholar] [CrossRef]
- Jamil, M.; Haq, I.U.; Mirza, B.; Qayyum, M. Isolation of antibacterial compounds from Quercus dilatata. through bioassay guided fractionation. Ann. Clin. Microb. Anti. 2012, 11, 11–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, S.K.; Basukala, P.; Basukala, O.; Parajuli, K.; Pokhrel, B.M.; Rijal, B.P. Detection of biofilm production and antibiotic resistance pattern in clinical isolates from indwelling medical devices. Curr. Microbiol. 2015, 70, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Naskar, A.; Khan, H.; Sarkar, R.; Kumar, S.; Halder, D.; Jana, S. Anti-biofilm activity and food packaging application of room temperature solution process based polyethylene glycol capped Ag-ZnO-graphene nanocomposite. Mater. Sci. Eng. C 2018, 91, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Khoushika, R.; Brindha, D. Evaluation of the antityrosinase and antioxidant potential of zinc oxide nanoparticles synthesized from the brown seaweed-Turbinaria conoides. Int. J. Appl. Pharm. 2017, 9, 116–120. [Google Scholar] [CrossRef] [Green Version]
- Chuysinuan, P.; Pavasant, P.; Supaphol, P. Preparation and characterization of Caffeic Acid-grafted Electrospun Poly (l-Lactic Acid) fiber mats for biomedical applications. ACS Appl. Mater. Interfaces 2012, 4, 3031–3040. [Google Scholar] [CrossRef]
- Thanyacharoen, T. The chemical composition and antioxidant and release properties of a black rice (Oryza sativa L.)-loaded chitosan and polyvinyl alcohol composite. J. Mol. Liq. 2017, 248, 1065–1070. [Google Scholar] [CrossRef]





| CoCl2 (%) | PMMA/ABS Conc. (%) | Impact Strength (J.mol−1) | Tensile Strength (MPa) | Elastic Modulus (GPa) | Elongation at Break (%) |
|---|---|---|---|---|---|
| 0 | 80:20 | 35 | 16.5 | 3.27 | 1.21 |
| 1.0 | 26 | 16.1 | 3.13 | 1.41 | |
| 2.5 | 36 | 15 | 2.88 | 1.53 | |
| 5.0 | 39 | 14 | 2.81 | 1.79 | |
| 7.5 | 43 | 12 | 2.58 | 2.15 | |
| 10.0 | 47 | 11 | 2.54 | 2.62 |
| Composite Materials | Zone of Inhibition (mm) | |||||
|---|---|---|---|---|---|---|
| S. aureus | S.pyogenes | B. subtilis | E. coli | S. typhi | P. aeruginosa | |
| PMMA/ABS blend | 20 ± 1.5 | 14 ± 1.5 | 18 ± 1.5 | 19 ± 1.15 | 13 ± 1.25 | 17 ± 1.15 |
| Blend + 2.5% CoCl2 | 24 ± 1.85 | 19 ± 1.35 | 23 ± 1.25 | 21 ± 1.50 | 19 ± 1.35 | - |
| Blend + 5% CoCl2 | 26 ± 1.45 | 25 ± 1.50 | 26 ± 1.45 | 23 ± 1.35 | 26 ± 1.25 | - |
| Blend + 7.5% CoCl2 | 28 ± 1.50 | 27 ± 1.20 | 31 ± 1.50 | 22 ± 1.25 | 29 ± 1.35 | 28 ± 1.50 |
| Blend + 10% CoCl2 | 31 ± 1.40 | 28 ± 1.30 | 30 ± 1.25 | 20 ± 1.45 | 31 ± 1.25 | 29 ± 1.05 |
| Cefixime | 33 ± 1.5 | 31 ± 1.0 | 35 ± 1.15 | 29 ± 0.5 | 36 ± 1.0 | 31 ± 2.25 |
| Composite Materials | Antioxidant Activity | ||
|---|---|---|---|
| DPPH (%) | ABTS (%) | FRAP (%) | |
| PMMA/ABS blend | 55 ± 1.25 | 27 ± 2.15 | 0.15 |
| Blend + 2.5% CoCl2 | 59 ± 1.5 | 30 ± 2.25 | 0.19 |
| Blend + 5% CoCl2 | 66 ± 1.5 | 44 ± 1.50 | 0.64 |
| Blend + 7.5% CoCl2 | 78 ± 2.0 | 58 ± 1.25 | 0.78 |
| Blend + 10% CoCl2 | 89 ± 1.0 | 65 ± 2.10 | 0.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zia, M.A.; Khosa, M.K.; Noor, A.; Qayyum, S.; Shakir, M.S. PMMA/ABS/CoCl2 Composites for Pharmaceutical Applications: Thermal, Antimicrobial, Antibiofilm, and Antioxidant Studies. Molecules 2022, 27, 7669. https://doi.org/10.3390/molecules27227669
Zia MA, Khosa MK, Noor A, Qayyum S, Shakir MS. PMMA/ABS/CoCl2 Composites for Pharmaceutical Applications: Thermal, Antimicrobial, Antibiofilm, and Antioxidant Studies. Molecules. 2022; 27(22):7669. https://doi.org/10.3390/molecules27227669
Chicago/Turabian StyleZia, Muhammad Abid, Muhammad Kaleem Khosa, Awal Noor, Sadaf Qayyum, and Muhammad Shabbir Shakir. 2022. "PMMA/ABS/CoCl2 Composites for Pharmaceutical Applications: Thermal, Antimicrobial, Antibiofilm, and Antioxidant Studies" Molecules 27, no. 22: 7669. https://doi.org/10.3390/molecules27227669
APA StyleZia, M. A., Khosa, M. K., Noor, A., Qayyum, S., & Shakir, M. S. (2022). PMMA/ABS/CoCl2 Composites for Pharmaceutical Applications: Thermal, Antimicrobial, Antibiofilm, and Antioxidant Studies. Molecules, 27(22), 7669. https://doi.org/10.3390/molecules27227669