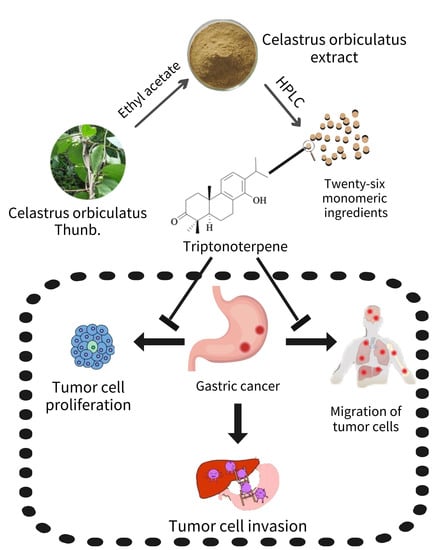
Triptonoterpene, a Natural Product from Celastrus orbiculatus Thunb, Has Biological Activity against the Metastasis of Gastric Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Triptonoterpene Inhibits the Viability of Gastric Cancer Cells
2.2. Triptonoterpene Inhibits the Proliferation of Gastric Cancer Cells
2.3. Triptonoterpene Inhibited the Adhesion of Gastric Cancer Cells to Cell Matrix
2.4. Triptonoterpene Inhibits the Migration of Gastric Cancer Cells
2.5. Triptonoterpene Inhibits the Invasion of Gastric Cancer Cells
2.6. Triptonoterpene Inhibits the Dynamic Migration of the Gastric Cancer Cells
2.7. Triptonoterpene Affects the Expression of the EMT-Related Proteins in Gastric Cancer Cells
2.8. Triptonoterpene Affects the Expression of the MMP-Related Proteins in the Gastric Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Drug
4.2. Reagents
4.3. Cell Culture
4.4. Cell Viability Assay
4.5. Colony Formation Assay
4.6. Cell Adhesion Assay
4.7. Wound Healing Assay
4.8. Transwell Chamber Assay
4.9. Operetta CLS High-Content Cell Dynamic Tracking Assay
4.10. Western Blot Analysis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thrift, A.P.; Nguyen, T.H. Gastric Cancer Epidemiology. Gastrointest. Endosc. Clin. North Am. 2021, 31, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H. Long-term survival rates of cancer patients achieved by the end of the 20th century: A period analysis. Lancet 2002, 360, 1131–1135. [Google Scholar] [CrossRef]
- Ilic, M.; Ilic, I. Epidemiology of stomach cancer. World J. Gastroenterol. 2022, 28, 1187–1203. [Google Scholar] [CrossRef]
- Lee, B.L.; Lee, H.S.; Jung, J.; Cho, S.J.; Chung, H.Y.; Kim, W.H.; Jin, Y.W.; Kim, C.S.; Nam, S.Y. Nuclear factor-kappaB activation correlates with better prognosis and Akt activation in human gastric cancer. Clin. Cancer Res. 2005, 11, 2518–2525. [Google Scholar] [CrossRef] [Green Version]
- Riihimäki, M.; Hemminki, A.; Sundquist, K.; Sundquist, J.; Hemminki, K. Metastatic spread in patients with gastric cancer. Oncotarget 2016, 7, 52307–52316. [Google Scholar] [CrossRef] [Green Version]
- Paul, C.D.; Mistriotis, P.; Konstantopoulos, K. Cancer cell motility: Lessons from migration in confined spaces. Nat. Rev. Cancer 2017, 17, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.; Lai, Z.; Lin, J. Anticancer Properties of Traditional Chinese Medicine. Comb. Chem. High Throughput. Screen. 2017, 20, 423–429. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Q.; Shao, Y.; Yin, S.; Liu, C.; Liu, Y.; Wang, R.; Wang, T.; Qiu, Y.; Yu, H. Anticancer activities of TCM and their active components against tumor metastasis. Biomed. Pharmacother. 2021, 133, 111044. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shen, W.; Pei, B.; Wang, X.; Sun, D.; Li, Y.; Xiu, L.; Liu, X.; Lu, Y.; Zhang, X.; et al. Decoction reverses MNNG-induced precancerous lesions of gastric carcinoma in vivo and vitro: Regulation of apoptosis through NF-κB pathway. Biomed. Pharmacother. 2018, 108, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhu, Y.; Su, Y.; Yu, M.; Xu, X.; Wang, C.; Zhang, S.; Xia, L. Taurochenodeoxycholic acid inhibits the proliferation and invasion of gastric cancer and induces its apoptosis. J. Food Biochem. 2022, 46, e13866. [Google Scholar] [CrossRef]
- Dai, X.; Liu, D.; Liu, M.; Zhang, X.; Wang, W.; Jin, F.; Qian, Y.; Wang, X.; Zhao, J.; Wu, Y.; et al. Anti-metastatic Efficacy of Traditional Chinese Medicine (TCM) Ginsenoside Conjugated to a VEFGR-3 Antibody on Human Gastric Cancer in an Orthotopic Mouse Model. Anticancer Res. 2017, 37, 979–986. [Google Scholar]
- Feng, G.; Chu, Z.; Wang, H.; Liu, Y.; Zhu, F. Celastrus orbiculatus extract inhibits the invasion and migration of human gastric cancer cells in a hypoxia microenvironment. Anticancer Agents Med Chem. 2022, 22, 3125–3135. [Google Scholar] [PubMed]
- Wang, H.; Chu, Z.; Ou, S.; Ni, T.; Dai, X.; Zhang, X.; Liu, Y. Celastrus orbiculatus Extract Inhibits the Epithelial-Mesenchymal Transition Process by Transforming Growth Factor-β Signaling Pathway in Gastric Cancer. Anticancer Agents Med Chem. 2022, 22, 2282–2291. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.D.; Hu, L.; Li, P.; Zhang, M.; Liu, Y.Q. Effects of Celastrus orbiculatus on Epithelial Mesenchymal Transition in Gastric Mucosal Epithelial Cells by Inhibiting Lgr5 Expression from Rats with Gastric Precancerous Lesions. Am. J. Chin. Med. 2018, 46, 1129–1143. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Qian, Y.; Dai, X.; Yang, L.; Chen, J.; Guo, S.; Hisamitsu, T. Antimetastatic effects of Celastrus orbiculatus on human gastric adenocarcinoma by inhibiting epithelial-mesenchymal transition and NF-κB/snail signaling pathway. Integr. Cancer Ther. 2015, 14, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Shan, T.Z.; Xu, J.J.; Chen, W.J.; Miao, L.; Lv, M.Y.; Tao, L.; Liu, Y.Q. Cytotoxic abietane and kaurane diterpenoids from Celastrus Orbiculatus. J. Nat. Med. 2019, 73, 841–846. [Google Scholar] [CrossRef]
- Chu, Z.W.; Shi, X.; Chen, G.Y.; He, X.J.; Qian, Y.Y.; Wang, H.B.; Tao, L.; Liu, Y.Q.; Jiang, W.; Chen, J. COE Inhibits Vasculogenic Mimicry by Targeting EphA2 in Hepatocellular Carcinoma, a Research Based on Proteomics Analysis. Front. Pharmacol. 2021, 12, 619732. [Google Scholar] [CrossRef]
- Qian, Y.Y.; Zhang, H.; Hou, Y.; Yuan, L.; Li, G.Q.; Guo, S.Y.; Hisamits, T.; Liu, Y.Q. Celastrus orbiculatus extract inhibits tumor angiogenesis by targeting vascular endothelial growth factor signaling pathway and shows potent antitumor activity in hepatocarcinomas in Vitro and in Vivo. Chin. J. Integr. Med. 2012, 18, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, D.; Guo, S.; Sunagawa, M.; Hisamitsu, T.; Liu, Y. Anti-invasive effects of Celastrus Orbiculatus extract on interleukin-1 beta and tumour necrosis factor-alpha combination-stimulated fibroblast-like synoviocytes. BMC Complement. Altern. Med. 2014, 14, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Qian, Y.; Liu, Y.; Li, G.; Cui, P.; Zhu, Y.; Ma, H.; Ji, X.; Guo, S.; Tadashi, H. Celastrus orbiculatus extract induces mitochondrial-mediated apoptosis in human hepatocellular carcinoma cells. J. Tradit. Chin. Med. 2012, 32, 621–626. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Liu, Y.; Wang, M.; Qian, Y.; Dai, X.; Zhu, Y.; Chen, J.; Guo, S.; Hisamitsu, T. Celastrus orbiculatus extract triggers apoptosis and autophagy via PI3K/Akt/mTOR inhibition in human colorectal cancer cells. Oncol. Lett. 2016, 12, 3771–3778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Gu, H.; Feng, J.; Qian, Y.; Yang, L.; Jin, F.; Wang, X.; Chen, J.; Shi, Y.; Lu, S. Celastrus orbiculatus extract suppresses the epithelial-mesenchymal transition by mediating cytoskeleton rearrangement via inhibition of the Cofilin 1 signaling pathway in human gastric cancer. Oncol. Lett. 2017, 14, 2926–2932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Zhou, Y.; Yao, Q.; Liu, W.; Xiang, L.; Ni, T.; Dai, X.; Liu, Y. Celastrus Orbiculatus Extract Potentiates the Sensitivity of Cisplatin Via Caspase-Depenent Apoptosis in Gastric Cancer. Anticancer Agents Med. Chem. 2018, 18, 2206–2211. [Google Scholar] [CrossRef]
- Wei, S.C.; Yang, J. Forcing through Tumor Metastasis: The Interplay between Tissue Rigidity and Epithelial-Mesenchymal Transition. Trends Cell Biol. 2016, 26, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Friedl, P.; Wolf, K. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat. Rev. Cancer 2003, 3, 362–374. [Google Scholar] [CrossRef]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [Green Version]
- Cho, E.S.; Kang, H.E.; Kim, N.H.; Yook, J.I. Therapeutic implications of cancer epithelial-mesenchymal transition (EMT). Arch. Pharm. Res. 2019, 42, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal tran-sitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Uchikado, Y.; Okumura, H.; Ishigami, S.; Setoyama, T.; Matsumoto, M.; Owaki, T.; Kita, Y.; Natsugoe, S. Increased Slug and decreased E-cadherin expression is related to poor prognosis in patients with gastric cancer. Gastric Cancer 2011, 14, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Zan, K.; Chen, X.; Wang, Q.; Cao, L. Studies on the chemical constituents of the stems of Ampelopsis nanensis. Chin. Herb. Med. 2007, 10, 1455–1457. [Google Scholar]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Luo, Y.; Hu, Y.; Feng, X.; Feng, J.; Chu, Z.; Ou, S.; Dai, X.; Wang, X.; Liu, Y. Triptonoterpene, a Natural Product from Celastrus orbiculatus Thunb, Has Biological Activity against the Metastasis of Gastric Cancer Cells. Molecules 2022, 27, 8005. https://doi.org/10.3390/molecules27228005
Wang H, Luo Y, Hu Y, Feng X, Feng J, Chu Z, Ou S, Dai X, Wang X, Liu Y. Triptonoterpene, a Natural Product from Celastrus orbiculatus Thunb, Has Biological Activity against the Metastasis of Gastric Cancer Cells. Molecules. 2022; 27(22):8005. https://doi.org/10.3390/molecules27228005
Chicago/Turabian StyleWang, Haibo, Yuanyuan Luo, Yaqi Hu, Xinyi Feng, Jun Feng, Zewen Chu, Shiya Ou, Xiaojun Dai, Xiaoqing Wang, and Yanqing Liu. 2022. "Triptonoterpene, a Natural Product from Celastrus orbiculatus Thunb, Has Biological Activity against the Metastasis of Gastric Cancer Cells" Molecules 27, no. 22: 8005. https://doi.org/10.3390/molecules27228005
APA StyleWang, H., Luo, Y., Hu, Y., Feng, X., Feng, J., Chu, Z., Ou, S., Dai, X., Wang, X., & Liu, Y. (2022). Triptonoterpene, a Natural Product from Celastrus orbiculatus Thunb, Has Biological Activity against the Metastasis of Gastric Cancer Cells. Molecules, 27(22), 8005. https://doi.org/10.3390/molecules27228005