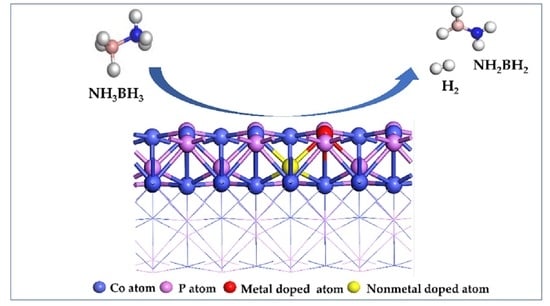
Theoretical Study of Hydrogen Production from Ammonia Borane Catalyzed by Metal and Non-Metal Diatom-Doped Cobalt Phosphide
Abstract
:1. Introduction
2. Calculation Methods
3. Results and Discussions
3.1. Adsorption of NH3BH3 on the Surface of CoP and Its Doped Catalysts
3.2. Hydrogen Evolution Mechanism of NH3BH3 on the Surface of the Catalyst
3.3. Performance Calculation of Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, L.; Sun, Z.; Zhang, L. Progress in Global Gas Hydrate Development and Production as a New Energy Resource. Acta Geol. Sin. 2019, 93, 731–755. [Google Scholar] [CrossRef]
- Yang, Z. Research on New Energy-saving and Efficient Utilization of Buildings Based on Renewable Energy. IOP Conf. Ser. Earth Environ. Sci. 2020, 514, 042016. [Google Scholar] [CrossRef]
- Manousiouthakis, V.; Cabezas, H. Editorial Overview: Hydrogen Energy; Sources and Various Issues. Curr. Opin. Chem. Eng. 2018, 21, i–iii. [Google Scholar] [CrossRef]
- Zhan, T.; Bie, R.; Shen, Q.; Lin, L.; Wu, A.; Dong, P. Application of Electrolysis Water Hydrogen Production in the Field of Renewable Energy Power Generation. IOP Conf. Ser. Earth Environ. Sci. 2020, 598, 012088. [Google Scholar] [CrossRef]
- Bajus, S.; Agel, F.; Kusche, M.; Ní Bhriaina, N.; Wasserscheid, P. Alkali hydroxide-modi fied Ru/gamma-Al2O3 catalysts for am-monia decomposition. Appl. Catal. A 2016, 510, 189–195. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, F.; Zeng, X.; Yao, G.; Jing, Z. A novel method for producing hydrogen from water with Fe enhanced by HS−under mild hydrothermal conditions. Int. J. Hydrogen Energy 2013, 38, 760–768. [Google Scholar] [CrossRef]
- Xin, Y.; Sun, B.; Zhu, X.; Yan, Z.; Zhao, X.; Sun, X.; Takayuki, O. Characteristics and pathways of hydrogen produced by pulsed discharge in ethanol-water mixtures. Int. J. Hydrogen Energy 2020, 45, 1588–1596. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Yang, J. Proposed photosynthesis method for producing hydrogen from dissociated water molecules using incident near-infrared light. Phys. Rev. Lett. 2014, 112, 018301. [Google Scholar] [CrossRef] [PubMed]
- Rossin, A.; Peruzzini, M. Ammonia-Borane and Amine-Borane Dehydrogenation Mediated by Complex Metal Hydrides. Chem. Rev. 2016, 116, 8848–8872. [Google Scholar] [CrossRef]
- Wu, H.; Cheng, Y.J.; Fan, Y.P.; Lu, X.M.; Li, L.X.; Liu, B.Z.; Li, B.J.; Lu, S.Y. Metal-catalyzed hydrolysis of ammonia borane: Mechanism, catalysts, and challenges. Int. J. Hydrogen Energy 2020, 45, 30325–30340. [Google Scholar] [CrossRef]
- Demirci, U.B. Ammonia borane, a material with exceptional properties for chemical hydrogen storage. Int. J. Hydrogen Energy 2017, 42, 9978–10013. [Google Scholar] [CrossRef]
- Staubitz, A.; Robertson, A.P.M.; Manners, I. Ammonia-borane and related compounds as dihydrogen sources. Chem. Rev. 2010, 110, 4079–4124. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Miyawaki, K.; Yamashita, H. Ru and Ru-Ni Nanoparticles on TiO2 Support as Extremely Active Catalysts for Hydrogen Production from Ammonia-Borane. ACS Catal. 2016, 6, 3128–3135. [Google Scholar] [CrossRef]
- Blaquiere, N.; Diallo-Garcia, S.; Gorelsky, S.I.; Black, D.A.; Fagnou, K. Ruthenium-catalyzed dehydrogenation of ammo nia boranes. J. Am. Chem. Soc. 2008, 130, 14034–14035. [Google Scholar] [CrossRef]
- Chen, G.Z.; Desinan, S.; Rosei, R.; Rosei, F.; Ma, D.L. Hollow ruthenium nanoparticles with small dimensions derived from Ni@ Ru core@ shell structure: Synthesis and enhanced catalytic dehydrogenation of ammonia borane. Chem. Comun. 2012, 48, 8009–8011. [Google Scholar] [CrossRef]
- Huo, J.R.; Fu, L.; Zhao, C.X.; He, C.Z. Hydrogen generation of ammonia borane hydrolysis catalyzed by Fe22@ Co58 core-shell structure. Chin. Chem. Lett. 2021, 32, 2269–2273. [Google Scholar] [CrossRef]
- Zhang, J.P.; Li, J.; Yang, L.J.; Li, R.; Zhang, F.M.; Dong, H. Efficient hydrogen production from ammonia borane hydrolysis cat alyzed by TiO2-supported RuCo catalysts. Int. J. Hydrogen Energy 2021, 46, 3964–3973. [Google Scholar] [CrossRef]
- Alpaydin, C.Y.; Gulbay, S.K.; Colpan, C.O. A review on the catalysts used for hydrogen production from ammonia borane. Int. J. Hydrogen Energy 2020, 45, 3414–3434. [Google Scholar] [CrossRef]
- Xu, P.; Lu, W.W.; Zhang, J.J.; Zhang, L. Efficient hydrolysis of ammonia borane for hydrogen evolution catalyzed by plasmonic Ag@ Pd core–shell nanocubes. ACS Sustain. Chem. Eng. 2020, 8, 12366–12377. [Google Scholar] [CrossRef]
- Liao, J.Y.; Feng, Y.F.; Zhang, X.B.; Huang, S.Q.; Liu, M.W.; Liu, Q.B.; Li, H. CuO-Co3O4 Composite Nanoplatelets for Hydrolyzing Ammonia Borane. ACS Appl. Nano Mater. 2021, 4, 7640–7649. [Google Scholar] [CrossRef]
- Feng, Y.F.; Zhang, X.F.; Shao, Y.X.; Chen, X.D.; Wang, H.Z.; Li, J.H.; Wu, M.; Dong, H.F.; Liu, Q.B.; Li, H. Modulating the Acidic Properties of Mesoporous Mox–Ni0.8Cu0.2O Nanowires for Enhanced Catalytic Performance toward the Methanolysis of Ammonia Borane for Hydrogen Production. ACS Appl. Mater. Interfaces 2022, 14, 27979–27993. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.Y.; Shao, Y.X.; Feng, Y.F.; Zhang, J.; Song, C.X.; Zeng, W.; Tang, J.T.; Dong, H.F.; Liu, Q.B.; Li, H. Interfacial Charge Transfer Induced Dual-active-sites of Heterostructured Cu0.8Ni0.2WO4 Nanoparticles in Ammonia Borane Methanolysis for Fast Hydrogen Production. Appl. Catal. B Environ. 2023, 320, 121973. [Google Scholar] [CrossRef]
- Feng, Y.F.; Shao, Y.X.; Chen, X.D.; Zhang, Y.D.; Liu, Q.B.; He, M.Y.; Li, H. Sea-Urchin-like Hollow CuMoO4–CoMoO4 Hybrid Microspheres, a Noble-Metal-like Robust Catalyst for the Fast Hydrogen Production from Ammonia Borane. ACS Appl. Energy Mater. 2021, 4, 633–642. [Google Scholar] [CrossRef]
- Liao, J.Y.; Wu, Y.J.; Shao, Y.X.; Feng, Y.F.; Zhang, X.F.; Zhang, W.L.; Li, J.H.; Wu, M.; Dong, H.F.; Liu, Q.B.; et al. Ammonia Borane Methanolysis for Hydrogen Evolution on Cu3Mo2O9/NiMoO4 Hollow Microspheres. Chem. Eng. J. 2022, 449, 137755. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, J.Q.; Cui, W.; Jiang, P.; Cheng, N.Y.; Asiri, A.M.; Sun, X.P. Carbon nanotubes decorated with CoP nanocrystals: A highly active non-noble-metal nanohybrid electrocatalyst for hydrogen evolution. Angew. Chem. Int. Ed. 2014, 53, 6710–6714. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, R.; Lu, W.B.; He, L.B.; Jiang, X.; Asiri, A.M.; Sun, X.P. Fe-Doped CoP Nanoarray: A Monolithic Multifunctional Catalyst for Highly Efficient Hydrogen Generation. Adv. Mater. 2017, 29, 1602441. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.R.; Yu, X.; Liu, X.; Teng, C.L.; Du, Y.S.; Wu, Q. Contrallable synthesis of peony-like porous Mn-CoP nanorod electrocatalyst for highly efficient hydrogen evolution in acid and alkaline. J. Colloid Interface Sci. 2020, 577, 379–387. [Google Scholar] [CrossRef]
- Ren, Y.C.; Li, Z.R.; Deng, B.; Ye, C.; Zhang, L.C.; Wang, Y.; Li, T.S.; Liu, Q.; Cui, G.W.; Asiri, A.M.; et al. Superior hydrogen evolution electrocatalysis enabled by CoP nanowire array on graphite felt. Int. J. Hydrogen Energy 2022, 47, 3580–3586. [Google Scholar] [CrossRef]
- Zhu, D.D.; Wang, L.; Qiao, M.; Liu, J.L. Phosphate ion functionalized CoP nanowire arrays for efficient alkaline hydrogen evolution. Chem. Commun. 2020, 56, 7159–7162. [Google Scholar] [CrossRef]
- Jing, Y.Q.; Liu, H.L.; Yang, R.J.; Chen, J.; Dai, H.T.; Liu, C.L.; Zhang, X.D. Mesoporous CoP nanowire arrays for hydrogen evolution. ACS Appl. Nano Mater. 2019, 2, 5922–5930. [Google Scholar] [CrossRef]
- Ma, L.Z.; Guo, J.X. S-doped CoP nanoneedles assembled urchin-like structure for efficient water oxidation. Mater. Lett. 2022, 307, 131005. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, X.; Xue, H.G.; Feng, L.G. A nitrogen-doped CoP nanoarray over 3D porous Co foam as an efficient bifunctional electrocatalyst for overall water splitting. J. Mater. Chem. A 2019, 7, 13242–13248. [Google Scholar] [CrossRef]
- Zhang, R.; Tang, C.; Kong, R.M.; Du, G.; Asiri, A.M.; Chen, L.; Sun, X.P. Al-Doped CoP nanoarray: A durable water-splitting electrocatalyst with superhigh activity. Nanoscale 2017, 9, 4793–4800. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Li, S.S.; Yoshida, A.; Sirisomboonchai, S.; Tang, K.Y.; Zuo, Z.J.; Hao, X.G.; Abudula, A.; Guan, G.Q. Mn doped CoP nanoparticle clusters: An efficient electrocatalyst for hydrogen evolution reaction. Catal. Sci. Technol. 2018, 8, 4407–4412. [Google Scholar] [CrossRef]
- Pan, Y.; Sun, K.A.; Lin, Y.; Cao, X.; Cheng, Y.S.; Liu, S.J.; Zeng, L.Y.; Cheong, W.C.; Zhao, D.; Wu, K.L.; et al. Electronic structure and d-band center control engineering over M-doped CoP (M = Ni, Mn, Fe) hollow polyhedron frames for boosting hydrogen production. Nano Energy 2019, 56, 411–419. [Google Scholar] [CrossRef]
- Wang, X.Q.; Chen, Y.F.; Yu, B.; Wang, Z.G.; Wang, H.Q.; Sun, B.C.; Li, W.X.; Yang, D.X.; Zhang, W.L. Hierarchically Porous W-Doped CoP Nanoflake Arrays as Highly Efficient and Stable Electrocatalyst for pH-Universal Hydrogen Evolution. Small 2019, 15, 1902613. [Google Scholar] [CrossRef]
- Zhou, Q.W.; Shen, Z.H.; Zhu, C.; Li, J.C.; Ding, Z.Y.; Wang, P.; Pan, F.; Zhang, Z.Y.; Ma, H.X.; Wang, S.Y.; et al. Nitrogen-Doped CoP Electrocatalysts for Coupled Hydrogen Evolution and Sulfur Generation with Low Energy Consumption. Adv. Mater. 2018, 30, 1800140. [Google Scholar] [CrossRef]
- Ma, X.C.; He, Y.Y.; Zhang, D.X.; Chen, M.J.; Ke, S.C.; Yin, Y.X.; Chang, G.G. Cobalt-Based MOF-Derived CoP/Hierarchical Porous Carbon (HPC) Composites as Robust Catalyst for Efficient Dehydrogenation of Ammonia-Borane. ChemistrySelect 2020, 5, 2190–2196. [Google Scholar] [CrossRef]
- Wu, H.; Cheng, Y.J.; Wang, B.Y.; Wang, Y.; Wu, M.; Li, W.D.; Liu, B.Z.; Lu, S.Y. Carbon dots-confined CoP-CoO nanoheterostructure with strong interfacial synergy triggered the robust hydrogen evolution from ammonia borane. J. Energy Chem. 2021, 57, 198–205. [Google Scholar] [CrossRef]
- Asim, M.; Zhang, S.G.; Wang, Y.T.; Maryam, B.; Sajid, M.; Shi, C.X.; Pan, L.; Zhang, X.W.; Zou, J.J. Self-supporting NiCoP for hydrogen generation via hydrolysis of ammonia borane. Fuel 2022, 318, 123544. [Google Scholar] [CrossRef]
- Chen, Y.F.; Feng, K.; Yuan, G.T.; Kang, Z.H.; Zhong, J. Highly efficient CoNiP nanoboxes on graphene oxide for the hydrolysis of ammonia borane. Chem. Eng. J. 2022, 428, 131219. [Google Scholar] [CrossRef]
- Pichler, C.M.; Gu, D.; Joshi, H.; Schuth, F. Influence of preparation method and doping of zirconium oxide onto the material characteristics and catalytic activity for the HDO reaction in nickel on zirconium oxide catalysts. J. Catal. 2018, 365, 367–375. [Google Scholar] [CrossRef]
- Chen, J.F.; Mao, Y.; Wang, H.F.; Hu, P. Theoretical Study of Heteroatom Doping in Tuning the Catalytic Activity of Graphene for Triiodide Reduction. ACS Catal. 2016, 6, 6804–6813. [Google Scholar] [CrossRef] [Green Version]
- Duan, L.; Wang, P.; Yu, X.C.; Han, X.; Chen, Y.N.; Zhao, P.; Li, D.L.; Yao, R. The synthesis and characterization of Ag-N dual-doped p-type ZnO: Experiment and theory. Phys. Chem. Chem. Phys. 2014, 16, 4092–4097. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Yan, Y.F.; Feng, S.; Chen, Y.R.; Li, L.X.; Zhang, L.; Yang, Z.Q. Hydrogen release mechanism and performance of ammonia borane catalyzed by transition metal catalysts Cu-Co/MgO(100). Energy Res. 2019, 43, 921–930. [Google Scholar] [CrossRef]
- Mu, J.S.; Xu, J.Y.; Zhou, C.M.; Wang, Q.; Wang, X.G.; Liu, Z.Y.; Zhao, X.J.; Yang, E.C. Self-Supported Oxygen and Molybdenum Dual-Doped Cobalt Phosphide Hierarchical Nanomaterials as Superior Bifunctional Electrocatalysts for Overall Water Splitting. ChemElectroChem 2021, 8, 103–111. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, S.S.A.; Órfão, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Halgren, T.A.; Lipscomb, W.N. The synchronous-transit method for determining reaction pathways and locating molecular transition states. Chem. Phys. Lett. 1977, 49, 225–232. [Google Scholar] [CrossRef]
- Bahamon, D.; Khalil, M.; Belabbes, A.; Alwahedi, Y.; Vega, L.F.; Polychronopoulou, K. A DFT study of the adsorption energy and electronic interactions of the SO2 molecule on a CoP hydrotreating catalyst. RSC Adv. 2021, 11, 2947–2957. [Google Scholar] [CrossRef]
- Cao, X.F.; Tan, Y.; Zheng, H.A.; Hu, J.; Chen, X.; Chen, Z. Effect of cobalt phosphide (CoP) vacancies on its hydrogen evolution activity via water splitting: A theoretical study. Phys. Chem. Chem. Phys. 2022, 24, 4644–4652. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.H.; Liu, C.; Zhong, X.L. Enhancing Electrocatalysis for Hydrogen Production over CoP Catalyst by Strain: A Density Functional Theory Study. Chem. Chem. Phys. 2019, 21, 9137. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.Y.; Ye, B.; Dai, H.J.; Qin, S.; Zhang, Y.Q.; Yang, Q.H. Ni-doped CoP/Co2P Nanospheres as Highly Efficient and StableHydrogen Evolution Catalysts in Acidic andAlkaline Mediums. J. Solid State Chem. 2021, 301, 122299. [Google Scholar] [CrossRef]
- Li, K.; Ding, H.L.; Zhou, J.J.; Wang, W.W.; Zhang, P.L. Nitrogen and Molybdenum Co-doped CoP Nnanohoneycombs on 3D Nitrogen-doped Porous Graphene as Enhanced Electrocatalyst for Oxygen Evolution Reaction. Int. J. Hydrogen Energy 2021, 46, 35585–35593. [Google Scholar] [CrossRef]
- Anjum, M.A.R.; Okyay, M.S.; Kim, M.; Lee, H.M.; Park, N.; Lee, J.S. Bifunctional Sulfur-doped Cobalt Phosphide Electrocatalyst Outperforms All-noble-metal Electrocatalysts in Alkaline Electrolyzer for Overall Water Splitting. Nano Energy 2018, 53, 286–295. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Zhu, C.M.; Zhou, Y.; Zhang, Y.; Xie, Y.D.; Lv, L.W.; Chen, W.L.; He, Y.Y.; Hu, Z.G. Design and synthesis Zn doped CoP/Co2P nanowire arrays for boosting hydrogen generation reaction. J. Solid State Chem. 2020, 285, 121231. [Google Scholar] [CrossRef]
- Zhang, Y.; Hui, Z.X.; Zhou, H.Y.; Zai, S.F.; Wen, Z.; Li, J.C.; Yang, C.C.; Jiang, Q. Ga Doping Enables Superior Alkaline Hydrogen Evolution Reaction Performances of CoP. Chem. Eng. J. 2022, 429, 132012. [Google Scholar] [CrossRef]
- Sun, R.; Bai, Y.; Luo, M.; Qu, M.X.; Wang, Z.H.; Sun, W.; Sun, K.N. Enhancing Polysulfide Confinement and Electrochemical Kinetics by Amorphous Cobalt Phosphide for Highly Efficient Lithium-Sulfur Batteries. ACS Nano 2020, 15, 739–750. [Google Scholar] [CrossRef]





| Pathway | B-H(1) | Co(3)-H(1) | N-H(2) | Co(3)-H(2) | Zn-H(2) | H(1)-H(2) | |
|---|---|---|---|---|---|---|---|
| I | M1Zn-S | 1.28 | 1.65 | 1.03 | -- | -- | 2.53 |
| TS1Zn-S | 1.81 | 1.47 | 1.02 | -- | -- | 2.19 | |
| M2Zn-S | 2.50 | 1.58 | 1.05 | -- | -- | 2.41 | |
| TS2Zn-S | 2.61 | 1.52 | 1.92 | -- | -- | 1.72 | |
| P1Zn-S | 3.77 | 1.62 | 3.66 | -- | -- | 0.88 | |
| II | M1Zn-S | -- | 1.65 | 1.03 | 3.45 | 3.03 | 2.53 |
| TS1Zn-S | -- | 1.47 | 1.02 | 3.29 | 2.66 | 2.19 | |
| M2Zn-S | -- | 1.58 | 1.05 | 3.31 | 2.96 | 2.41 | |
| TS3Zn-S | -- | 1.54 | 1.51 | 3.15 | 2.52 | 2.27 | |
| M3Zn-S | -- | 1.65 | 2.55 | 1.60 | 1.86 | 2.35 | |
| TS4Zn-S | -- | 1.63 | 3.58 | 1.59 | 3.05 | 1.49 | |
| P1Zn-S | -- | 1.62 | 3.66 | 1.61 | 3.86 | 0.09 | |
| III | M1Zn-S | 1.28 | 1.65 | 1.03 | 3.45 | 3.03 | 2.53 |
| TS5Zn-S | 1.78 | 1.62 | 1.50 | 2.78 | 2.37 | 2.75 | |
| M3Zn-S | 3.33 | 1.63 | 2.55 | 1.59 | 3.05 | 2.35 | |
| TS4Zn-S | 3.83 | 1.63 | 3.58 | 1.59 | 3.05 | 1.49 | |
| P1Zn-S | 3.77 | 1.62 | 3.66 | 1.61 | 3.86 | 0.88 | |
| IV | M1Zn-S | 1.28 | -- | 1.03 | -- | -- | 2.53 |
| TS6Zn-S | 2.30 | -- | 1.91 | -- | -- | 1.99 | |
| P1Zn-S | 3.77 | -- | 3.66 | -- | -- | 0.88 | |
| Pathway | Compound | Erel | Ea |
|---|---|---|---|
| kcal/mol | kcal/mol | ||
| pathway I | M1Zn-S | 0.00 | |
| TS1Zn-S | 22.71 | 22.71 | |
| M2Zn-S | −4.83 | ||
| TS2Zn-S | 37.21 | 39.56 | |
| P1Zn-S | −29.93 | ||
| pathway II | M1Zn-S | 0.00 | |
| TS1Zn-S | 22.71 | 22.71 | |
| M2Zn-S | −4.83 | ||
| TS3Zn-S | 37.21 | 42.04 | |
| M3Zn-S | −29.93 | ||
| TS4Zn-S | −20.20 | 9.73 | |
| P1Zn-S | −26.62 | ||
| pathway III | M1Zn-S | 0.00 | |
| TS5Zn-S | 28.80 | 28.80 | |
| M3Zn-S | −29.93 | ||
| TS4Zn-S | −20.20 | 9.73 | |
| P1Zn-S | −26.62 | ||
| pathway IV | M1Zn-S | 0.00 | |
| TS6Zn-S | 22.15 | 22.15 | |
| P1Zn-S | −26.62 |
| Pathway | Compound | CoP | CoPNi-N | CoPGa-N | CoPNi-S | CoPZn-S |
|---|---|---|---|---|---|---|
| pathway I | TS1 | 21.88 | 29.15 | 22.48 | 20.67 | 22.71 |
| TS2 | 51.65 | 44.87 | 65.63 | 45.16 | 39.56 | |
| pathway II | TS1 | 21.88 | 29.15 | 22.48 | 20.67 | 22.71 |
| TS3 | 31.35 | 35.81 | 23.18 | 19.14 | 42.04 | |
| TS4 | 0.57 | 5.27 | 6.67 | 7.40 | 9.73 | |
| pathway III | TS5 | 36.68 | 33.45 | 47.29 | 47.94 | 28.80 |
| TS4 | 0.57 | 5.27 | 6.67 | 7.40 | 9.73 | |
| pathway IV | TS6 | 52.02 | 27.11 | 44.19 | 48.48 | 22.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.-H.; Li, Q.-M.; Qi, S.-L.; Qin, H.-C.; Liang, X.-Q.; Li, L. Theoretical Study of Hydrogen Production from Ammonia Borane Catalyzed by Metal and Non-Metal Diatom-Doped Cobalt Phosphide. Molecules 2022, 27, 8206. https://doi.org/10.3390/molecules27238206
Li D-H, Li Q-M, Qi S-L, Qin H-C, Liang X-Q, Li L. Theoretical Study of Hydrogen Production from Ammonia Borane Catalyzed by Metal and Non-Metal Diatom-Doped Cobalt Phosphide. Molecules. 2022; 27(23):8206. https://doi.org/10.3390/molecules27238206
Chicago/Turabian StyleLi, Dong-Heng, Qiao-Mei Li, Shuang-Ling Qi, Hai-Chuan Qin, Xiao-Qin Liang, and Laicai Li. 2022. "Theoretical Study of Hydrogen Production from Ammonia Borane Catalyzed by Metal and Non-Metal Diatom-Doped Cobalt Phosphide" Molecules 27, no. 23: 8206. https://doi.org/10.3390/molecules27238206
APA StyleLi, D. -H., Li, Q. -M., Qi, S. -L., Qin, H. -C., Liang, X. -Q., & Li, L. (2022). Theoretical Study of Hydrogen Production from Ammonia Borane Catalyzed by Metal and Non-Metal Diatom-Doped Cobalt Phosphide. Molecules, 27(23), 8206. https://doi.org/10.3390/molecules27238206