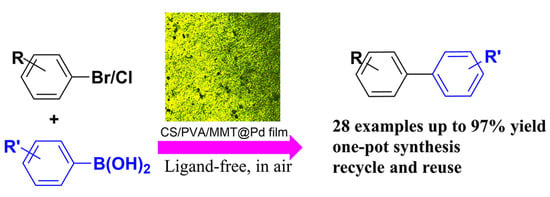
Preparation of a Montmorillonite-Modified Chitosan Film-Loaded Palladium Heterogeneous Catalyst and its Application in the Preparation of Biphenyl Compounds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Mechanical Properties of CS, PVA, CS/PVA, and CS/PVA/MMT Film
2.2. Dynamic Adsorption of the CS/PVA/MMT Film
2.3. Optimization of Reaction Conditions
2.4. Gram-Scale Synthesis Reaction
2.5. Catalyst Reuse
2.6. Characterization of Catalytic Materials
2.6.1. FTIR Characterization
2.6.2. Thermogravimetric Analyses
2.6.3. Polarization Microscopy Analyses
2.6.4. XRD Analysis
2.6.5. XPS Analysis
2.6.6. TEM Analysis
2.7. Discussion
3. Materials and Methods
3.1. Materials
3.2. Analytical Tests and Characterization
3.3. Preparation of Catalytic Materials
3.4. Adsorption Kinetic Experiments with Pd2+ (25 °C)
3.5. Testing of Mechanical Properties of Film
3.6. Suzuki Coupling Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jain, Z.J.; Gide, P.S.; Kankate, R.S. Biphenyls and their derivatives as synthetically and pharmacologically important aromatic structural moieties. Arab. J. Chem. 2017, 10, S2051–S2066. [Google Scholar] [CrossRef] [Green Version]
- Babkov, D.A.; Zhukowskaya, O.N.; Borisov, A.V.; Babkova, V.A.; Sokolova, E.V.; Brigadirova, A.A.; Litvinov, R.A.; Kolodina, A.A.; Morkovnik, A.S.; Sochnev, V.S.; et al. Towards multi-target antidiabetic agents: Discovery of biphenyl-benzimidazole conjugates as AMPK activators. Bioorganic Med. Chem. Lett. 2019, 29, 2443–2447. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, C.; Lee, J. Reduction of polycyclic compounds and biphenyls generated by pyrolysis of industrial plastic waste by using supported metal catalysts: A case study of polyethylene terephthalate treatment. J. Hazard. Mater. 2020, 392, 122464. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, V.I.; Antonov, D.Y.; Zhuravsky, R.P.; Vorontsov, E.V.; Starikova, Z.A. A novel class of bidentate ligands with a conformationally flexible biphenyl unit built into a planar chiral [2.2]paracyclophane backbone. Tetrahedron Lett. 2003, 44, 3801–3804. [Google Scholar] [CrossRef]
- Pan, B.; Ouyang, J.-S.; Zhang, Y.; Liang, H.; Ni, Q.; Chen, B.; Pu, X.; Jiang, L.; Cao, R.; Qiu, L. Iridium-catalyzed intramolecular asymmetric allylic etherification of salicylic acid derivatives with chiral-bridged biphenyl phosphoramidite ligands. Org. Chem. Front. 2021, 8, 4514–4519. [Google Scholar] [CrossRef]
- Thomas, A. Functional Materials: From Hard to Soft Porous Frameworks. Angew. Chem. Int. Ed. 2010, 49, 8328–8344. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Wu, X.; Chu, W.; Yin, S. Solution-processable, high current efficiency deep-blue organic light-emitting diodes based on novel biphenyl-imidazole derivatives. J. Mater. Chem. C 2020, 8, 11239–11251. [Google Scholar] [CrossRef]
- Cammidge, A.N.; Crépy, K.V.L. Application of the Suzuki Reaction as the Key Step in the Synthesis of a Novel Atropisomeric Biphenyl Derivative for Use as a Liquid Crystal Dopant. J. Org. Chem. 2003, 68, 6832–6835. [Google Scholar] [CrossRef]
- Alamro, F.S.; Tolan, D.A.; El-Nahas, A.M.; Ahmed, H.A.; El-Atawy, M.A.; Al-Kadhi, N.S.; Aziz, S.G.; Shibl, M.F. Wide Nematogenic Azomethine/Ester Liquid Crystals Based on New Biphenyl Derivatives: Mesomorphic and Computational Studies. Molecules 2022, 27, 4150. [Google Scholar] [CrossRef]
- Cammidge, A.N.; Crépy, K.V.L. The first asymmetric Suzuki cross-coupling reaction. Chem. Commun. 2000, 18, 1723–1724. [Google Scholar] [CrossRef]
- Suzuki, A. Organoborane coupling reactions (Suzuki coupling). Proc. Jpn. Acad. Ser. B 2004, 80, 359–371. [Google Scholar] [CrossRef] [Green Version]
- Tao, X.; Long, R.; Wu, D.; Hu, Y.; Qiu, G.; Qi, Z.; Li, B.; Jiang, R.; Xiong, Y. Anchoring Positively Charged Pd Single Atoms in Ordered Porous Ceria to Boost Catalytic Activity and Stability in Suzuki Coupling Reactions. Small 2020, 16, 2001782. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Sarina, S.; Jaatinen, E.; Jia, J.; Arnold, D.P.; Liu, H.; Zhu, H. Efficient photocatalytic Suzuki cross-coupling reactions on Au–Pd alloy nanoparticles under visible light irradiation. Green Chem. 2014, 16, 4272–4285. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Azarian, A.; Maham, M.; Ehsani, A. Synthesis of Au/Pd bimetallic nanoparticles and their application in the Suzuki coupling reaction. J. Ind. Eng. Chem. 2015, 21, 746–748. [Google Scholar] [CrossRef]
- Shaikh, N.M.; Sawant, A.D.; Bagihalli, G.B.; Challa, M.; Adimule, V.M. Highly active mixed Au–Pd nanoparticles supported on RHA silica through immobilised ionic liquid for suzuki coupling reaction. Top. Catal. 2022, 65, 1–10. [Google Scholar] [CrossRef]
- Gao, S.; Shang, N.; Feng, C.; Wang, C.; Wang, Z. Graphene oxide–palladium modified Ag–AgBr: A visible-light-responsive photocatalyst for the Suzuki coupling reaction. RSC Adv. 2014, 4, 39242–39247. [Google Scholar] [CrossRef]
- Bayan, R.; Karak, N. Photo-assisted synthesis of a Pd–Ag@ CQD nanohybrid and its catalytic efficiency in promoting the suzuki–miyaura cross-coupling reaction under ligand-free and ambient conditions. ACS Omega 2017, 2, 8868–8876. [Google Scholar] [CrossRef] [Green Version]
- Gorji, S.; Ghorbani-Vaghei, R. Ag nanoparticles stabilized on basalt fibers as a novel, stable, and reusable catalyst for Suzuki–Miyaura coupling reactions. Appl. Organomet. Chem. 2021, 35, e6018. [Google Scholar] [CrossRef]
- Colacot, T.J.; Qian, H.; Cea-Olivares, R.; Hernandez-Ortega, S. Synthesis, X-ray, spectroscopic and a preliminary Suzuki coupling screening studies of a complete series of dppfMX2 (M = Pt, Pd; X = Cl, Br, I). J. Organomet. Chem. 2001, 637, 691–697. [Google Scholar] [CrossRef]
- Li, X.; Zhao, X.; Zhang, J.; Zhao, Y. Assembly of a multilayer film and catalytic application in Suzuki cross-coupling reaction based on synergistic effects of a conjugated organometallic pyridyl Pt (C [triple bond, length as m-dash] C) 2 moiety with palladium. Chem. Commun. 2013, 49, 10004–10006. [Google Scholar] [CrossRef]
- Lee, E.-K.; Park, S.-A.; Woo, H.; Hyun Park, K.; Kang, D.W.; Lim, H.; Kim, Y.-T. Platinum single atoms dispersed on carbon nanotubes as reusable catalyst for Suzuki coupling reaction. J. Catal. 2017, 352, 388–393. [Google Scholar] [CrossRef]
- Alkabli, J.; Rizk, M.A.; Elshaarawy, R.F.; El-Sayed, W. Ionic chitosan Schiff bases supported Pd (II) and Ru (II) complexes; production, characterization, and catalytic performance in Suzuki cross-coupling reactions. Int. J. Biol. Macromol. 2021, 184, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Snieckus, V. Beyond directed ortho metalation: Ru-catalyzed CAr–O activation/cross-coupling reaction by amide chelation. J. Am. Chem. Soc. 2014, 136, 11224–11227. [Google Scholar] [CrossRef]
- Gooßen, L.J.; Paetzold, J. New Synthesis of Biaryls via Rh-Catalyzed Decarbonylative Suzuki-Coupling of Carboxylic Anhydrides with Arylboroxines. Adv. Synth. Catal. 2004, 346, 1665–1668. [Google Scholar] [CrossRef]
- Wang, S.-B.; Zhu, W.; Ke, J.; Lin, M.; Zhang, Y.-W. Pd–Rh nanocrystals with tunable morphologies and compositions as efficient catalysts toward Suzuki cross-coupling reactions. ACS Catal. 2014, 4, 2298–2306. [Google Scholar] [CrossRef]
- Zhou, J.; Hou, W.; Liu, X.; Astruc, D. Pd, Rh and Ru nanohybrid-catalyzed tetramethyldisiloxane hydrolysis for H 2 generation, nitrophenol reduction and Suzuki–Miyaura cross-coupling. Inorg. Chem. Front. 2022, 9, 1416–1422. [Google Scholar] [CrossRef]
- Rostamnia, S.; Alamgholiloo, H.; Liu, X. Pd-grafted open metal site copper-benzene-1,4-dicarboxylate metal organic frameworks (Cu-BDC MOF’s) as promising interfacial catalysts for sustainable Suzuki coupling. J. Colloid Interface Sci. 2016, 469, 310–317. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, X.; Sun, J.; Lin, S.; Qi, D.; Hong, R.; Li, D.; Xiao, X.; Jiang, J. Good Suzuki-coupling reaction performance of Pd immobilized at the metal-free porphyrin-based covalent organic framework. Microporous Mesoporous Mater. 2015, 214, 108–114. [Google Scholar] [CrossRef]
- Li, Y.; Pei, B.; Chen, J.; Bing, S.; Hou, L.; Sun, Q.; Xu, G.; Yao, Z.; Zhang, L. Hollow nanosphere construction of covalent organic frameworks for catalysis: (Pd/C)@TpPa COFs in Suzuki coupling reaction. J. Colloid Interface Sci. 2021, 591, 273–280. [Google Scholar] [CrossRef]
- Sun, X.; Li, S.; Cao, J.; Wang, Y.; Yang, W.; Zhang, L.; Liu, Y.; Qiu, J.; Tao, S. A Hierarchical-Structured Impeller with Engineered Pd Nanoparticles Catalyzing Suzuki Coupling Reactions for High-Purity Biphenyl. ACS Appl. Mater. Interfaces 2021, 13, 17429–17438. [Google Scholar] [CrossRef]
- Sahu, P.K.; Sahu, P.K.; Gupta, S.K.; Agarwal, D.D. Chitosan: An Efficient, Reusable, and Biodegradable Catalyst for Green Synthesis of Heterocycles. Ind. Eng. Chem. Res. 2014, 53, 2085–2091. [Google Scholar] [CrossRef]
- El Kadib, A. Chitosan as a Sustainable Organocatalyst: A Concise Overview. ChemSusChem 2015, 8, 217–244. [Google Scholar] [CrossRef] [PubMed]
- Molnár, Á. The use of chitosan-based metal catalysts in organic transformations. Coord. Chem. Rev. 2019, 388, 126–171. [Google Scholar] [CrossRef]
- Zheng, K.; Xiao, S.; Li, W.; Wang, W.; Chen, H.; Yang, F.; Qin, C. Chitosan-acorn starch-eugenol edible film: Physico-chemical, barrier, antimicrobial, antioxidant and structural properties. Int. J. Biol. Macromol. 2019, 135, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Zhang, C.; Wang, K.; Dou, B.; Song, Y.; Chen, H.; Xu, Y. Highly dispersed Ni/montmorillonite catalyst for glycerol steam reforming: Effect of Ni loading and calcination temperature. Appl. Therm. Eng. 2016, 109, 99–108. [Google Scholar] [CrossRef]
- Huang, W.J.; Liu, J.H.; She, Q.M.; Zhong, J.Q.; Christidis, G.E.; Zhou, C.H. Recent advances in engineering montmorillonite into catalysts and related catalysis. Catal. Rev. 2021, 63, 1–57. [Google Scholar] [CrossRef]
- Yang, S.; Huang, Z.; Wu, P.; Li, Y.; Dong, X.; Li, C.; Zhu, N.; Duan, X.; Dionysiou, D.D. Rapid removal of tetrabromobisphenol A by α-Fe2O3-x@Graphene@Montmorillonite catalyst with oxygen vacancies through peroxymonosulfate activation: Role of halogen and α-hydroxyalkyl radicals. Appl. Catal. B Environ. 2020, 260, 118129. [Google Scholar] [CrossRef]
- Zheng, K.; Lin Ren, Q.; Mo, R.; Deng, X.; Sun, B.; Wang, W.; Xiao, Z.; Qin, C. Adsorption of Cu2+ by modified chitosan microspheres and its application in homocoupling of arylboronic acid. Arab. J. Chem. 2022, 15, 104170. [Google Scholar] [CrossRef]
- Koosha, M.; Mirzadeh, H. Electrospinning, mechanical properties, and cell behavior study of chitosan/PVA nanofibers. J. Biomed. Mater. Res. Part A 2015, 103, 3081–3093. [Google Scholar] [CrossRef]
- Ghasemi Hamidabadi, H.; Rezvani, Z.; Nazm Bojnordi, M.; Shirinzadeh, H.; Seifalian, A.M.; Joghataei, M.T.; Razaghpour, M.; Alibakhshi, A.; Yazdanpanah, A.; Salimi, M.; et al. Chitosan-Intercalated Montmorillonite/Poly(vinyl alcohol) Nanofibers as a Platform to Guide Neuronlike Differentiation of Human Dental Pulp Stem Cells. ACS Appl. Mater. Interfaces 2017, 9, 11392–11404. [Google Scholar] [CrossRef]
- Noori, S.; Kokabi, M.; Hassan, Z.M. Nanoclay Enhanced the Mechanical Properties of Poly(Vinyl Alcohol)/Chitosan /Montmorillonite Nanocomposite Hydrogel as Wound Dressing. Procedia Mater. Sci. 2015, 11, 152–156. [Google Scholar] [CrossRef]
- Morisada, S.; Kim, Y.-H.; Ogata, T.; Marutani, Y.; Nakano, Y. Improved Adsorption Behaviors of Amine-Modified Tannin Gel for Palladium and Platinum Ions in Acidic Chloride Solutions. Ind. Eng. Chem. Res. 2011, 50, 1875–1880. [Google Scholar] [CrossRef]
- Lin, S.; Wei, W.; Wu, X.; Zhou, T.; Mao, J.; Yun, Y.-S. Selective recovery of Pd(II) from extremely acidic solution using ion-imprinted chitosan fiber: Adsorption performance and mechanisms. J. Hazard. Mater. 2015, 299, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.H.; Szeto, Y.S.; McKay, G. Intraparticle diffusion processes during acid dye adsorption onto chitosan. Bioresour. Technol. 2007, 98, 2897–2904. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Rao, X.; Zhang, Y.; Li, X.; Qiu, J.; Jin, Z. An Aerobic and Very Fast Pd/C-Catalyzed Ligand-Free and Aqueous Suzuki Reaction Under Mild Conditions. Eur. J. Org. Chem. 2013, 2013, 4345–4350. [Google Scholar] [CrossRef]
- Hong, K.; Sajjadi, M.; Suh, J.M.; Zhang, K.; Nasrollahzadeh, M.; Jang, H.W.; Varma, R.S.; Shokouhimehr, M. Palladium Nanoparticles on Assorted Nanostructured Supports: Applications for Suzuki, Heck, and Sonogashira Cross-Coupling Reactions. ACS Appl. Nano Mater. 2020, 3, 2070–2103. [Google Scholar] [CrossRef]
- Kim, S.; Jee, S.; Choi, K.M.; Shin, D.-S. Single-atom Pd catalyst anchored on Zr-based metal-organic polyhedra for Suzuki-Miyaura cross coupling reactions in aqueous media. Nano Res. 2021, 14, 486–492. [Google Scholar] [CrossRef]
- Moniriyan, F.; Sabounchei, S.J.; Yousefi, A.; Akhavan, O. Synthesis, morpho-structural properties, and catalytic performances of Pt-APA@ Fe3O4/GO nanocomposite based on magnetical graphene in C–C coupling reactions and photoinactivation of E. coli. J. Nanoparticle Res. 2021, 23, 1–16. [Google Scholar] [CrossRef]
- Kumar, L.M.; Ansari, R.M.; Bhat, B.R. Retracted: Catalytic activity of Fe (II) and Cu (II) PNP pincer complexes for Suzuki coupling reaction. Appl. Organomet. Chem. 2018, 32, e4054. [Google Scholar] [CrossRef]
- Pan, C.; Liu, M.; Zhang, L.; Wu, H.; Ding, J.; Cheng, J. Palladium catalyzed ligand-free Suzuki cross-coupling reaction. Catal. Commun. 2008, 9, 508–510. [Google Scholar] [CrossRef]
- Zhang, G. Efficient protocol for the phosphine-free Suzuki-Miyaura reaction catalyzed by palladium on carbon at room temperature. Synthesis 2005, 2005, 537–542. [Google Scholar] [CrossRef]
- Min, H.; Miyamura, H.; Kobayashi, S. N-Heterocyclic carbene coordinated heterogeneous Pd nanoparticles as catalysts for Suzuki–Miyaura coupling. Chem. Lett. 2016, 45, 837–839. [Google Scholar] [CrossRef]
- So, J.I.; Hwang, S.; Lee, M.Y.; Song, M.; Baeck, S.-H.; Shim, S.E.; Qian, Y. Evaluation of Nitrogen-Based Polymeric Heterogeneous Catalysts for the Suzuki–Miyaura Cross-Coupling Reaction in Water. ACS Appl. Polym. Mater. 2020, 2, 3122–3134. [Google Scholar] [CrossRef]
- Baran, T.; Inanan, T.; Menteş, A. Synthesis, characterization, and catalytic activity in Suzuki coupling and catalase-like reactions of new chitosan supported Pd catalyst. Carbohydr. Polym. 2016, 145, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Baran, T.; Yılmaz Baran, N.; Menteş, A. Sustainable chitosan/starch composite material for stabilization of palladium nanoparticles: Synthesis, characterization and investigation of catalytic behaviour of Pd@ chitosan/starch nanocomposite in Suzuki–Miyaura reaction. Appl. Organomet. Chem. 2018, 32, e4075. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M. Pd nanoparticles synthesized in situ with the use of Euphorbia granulate leaf extract: Catalytic properties of the resulting particles. J. Colloid Interface Sci. 2016, 462, 243–251. [Google Scholar] [CrossRef]
- Zheng, K.; Yang, F.; Huang, Z.; Zhan, Y.; Xiao, Z.; Li, W.; Wang, W.; Qin, C. Preparation of chitosan film-loaded palladium catalyst materials and their application in Suzuki coupling reactions. J. Mater. Res. Technol. 2022, 20, 3905–3917. [Google Scholar] [CrossRef]










| Entry | pH | Original Concentration (c0, mg/L) | Adsorption Time (min) | Residual Concentration (mg/L) | Absorption Percentage (%) | Absorption Capacity (qi, mg/g) |
|---|---|---|---|---|---|---|
| 1 | 3 | 100.00 | 300 | 5.09 | 94.91 | 94.91 |
| 2 | 2 | 100.00 | 300 | 37.82 | 62.18 | 62.18 |
| 3 | 1 | 100.00 | 300 | 60.09 | 39.91 | 39.91 |
| 4 | 3 | 20.15 | 300 | 0.99 | 95.09 | 19.16 |
| 5 | 3 | 40.76 | 300 | 1.22 | 97.01 | 39.54 |
| 6 | 3 | 60.02 | 300 | 1.61 | 97.31 | 58.41 |
| 7 | 3 | 80.07 | 300 | 3.73 | 95.34 | 76.34 |
| 8 | 3 | 100.00 | 10 | 74.25 | 25.75 | 25.75 |
| 9 | 3 | 100.00 | 20 | 58.3 | 41.70 | 41.7 |
| 10 | 3 | 100.00 | 30 | 45.22 | 54.78 | 54.78 |
| 11 | 3 | 100.00 | 60 | 36.02 | 63.98 | 63.98 |
| 12 | 3 | 100.00 | 90 | 32.16 | 67.84 | 67.84 |
| 13 | 3 | 100.00 | 120 | 24.11 | 75.89 | 75.89 |
| 14 | 3 | 100.00 | 180 | 15.02 | 84.98 | 84.98 |
| 15 | 3 | 100.00 | 240 | 7.86 | 92.14 | 92.14 |
| 16 | 3 | 100.00 | 360 | 5.11 | 94.89 | 94.89 |
| 17 a | 3 | 100.00 | 300 | 28.7 | 71.3 | 71.3 |
 | ||||||
|---|---|---|---|---|---|---|
| Entry | CS/PVA@Pd(mg) | Solvent (2 mL) | Base (1.2eq) | Time(h) | T/°C | Yield(%) |
| 1 | 10 | MeOH | Na2CO3 | 12 | 60 | 59 |
| 2 | 10 | EtOH | Na2CO3 | 12 | 60 | 55 |
| 3 | 10 | i-PrOH | Na2CO3 | 12 | 60 | 50 |
| 4 | 10 | Toluene | Na2CO3 | 12 | 60 | 42 |
| 5 | 10 | Acetone | Na2CO3 | 12 | 60 | 10 |
| 6 | 10 | CH3CN | Na2CO3 | 12 | 60 | 22 |
| 7 | 10 | CHCl3 | Na2CO3 | 12 | 60 | 13 |
| 8 | 10 | H2O | Na2CO3 | 12 | 60 | - |
| 9 | 10 | MeOH: H2O(1:1) | Na2CO3 | 12 | 60 | 86 |
| 10 | 10 | EtOH: H2O(1:1) | Na2CO3 | 12 | 60 | 88 |
| 11 | 10 | i-PrOH: H2O(1:1) | Na2CO3 | 12 | 60 | 80 |
| 12 | 10 | Toluene:H2O(1:1) | Na2CO3 | 12 | 60 | 34 |
| 13 | 10 | Acetone:H2O(1:1) | Na2CO3 | 12 | 60 | 28 |
| 14 | 10 | EtOH: H2O(2:1) | Na2CO3 | 12 | 60 | 92 |
| 15 | 10 | EtOH: H2O(4:1) | Na2CO3 | 12 | 60 | 91 |
| 16 | 10 | EtOH: H2O(1:2) | Na2CO3 | 12 | 60 | 87 |
| 17 | 10 | EtOH: H2O(2:1) | Li2CO3 | 12 | 60 | 75 |
| 18 | 10 | EtOH: H2O(2:1) | K2CO3 | 12 | 60 | 93 |
| 19 | 10 | EtOH: H2O(2:1) | Cs2CO3 | 12 | 60 | 95 |
| 20 | 10 | EtOH: H2O(2:1) | - | 12 | 60 | - |
| 21 | 10 | EtOH: H2O(2:1) | Cs2CO3 (1.5eq) | 12 | 60 | 95 |
| 22 | 10 | EtOH: H2O(2:1) | Cs2CO3 (2eq) | 12 | 60 | 94 |
| 23 | 10 | EtOH: H2O(2:1) | Cs2CO3 | 12 | 70 | 97 |
| 24 | 10 | EtOH: H2O(2:1) | Cs2CO3 | 12 | 80 | 96 |
| 25 | 10 | EtOH: H2O(2:1) | Cs2CO3 | 12 | 90 | 92 |
| 26 | 6 | EtOH: H2O(2:1) | Cs2CO3 | 12 | 70 | 89 |
| 27 | 8 | EtOH: H2O(2:1) | Cs2CO3 | 12 | 70 | 96 |
| 28 | 12 | EtOH: H2O(2:1) | Cs2CO3 | 12 | 70 | 97 |
| 29 | 10 | EtOH: H2O(2:1) | Cs2CO3 | 6 | 70 | 75 |
| 30 | 10 | EtOH: H2O(2:1) | Cs2CO3 | 8 | 70 | 95 |
| 31 | 10 | EtOH: H2O(2:1) | Cs2CO3 | 10 | 70 | 97 |
| 32 | 10 | EtOH: H2O(2:1) | Cs2CO3 | 16 | 70 | 95 |
 | |||
 |  |  |  |
| 3a, 97% | 3b, 90% | 3c, 95% | 3d, 93% |
 |  |  |  |
| 3e, 83% | 3f, 87% | 3g, 86% | 3h, 92% |
 |  |  |  |
| 3i, 68% | 3j, 85% | 3k, 88% | 3l, 78% |
 |  |  |  |
| 3m,52% | 3n, 86% | 3o, 92% | 3p, 90% |
 |  |  |  |
| 3q, 92% | 3r, 81 | 3s, 77% | 3t, 85% |
 |  |  |  |
| 3u, 96% | 3v, 86% | 3w, 67% | 3x, 83% |
 |  | ||
| 3y, 75% | 3z, 84% | ||
 | |||
 |  |  |  |
| 3a, 28% | 3b, 19% | 3c, 22% | 3d, 23% |
 |  |  |  |
| 3q, 25% | 3u, 30% | 3aa, 41% | 3ab, 37% |
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Catalyst | Solvent | Additive | Temp. (°C) | Time (h) | Yield (%) | Ref. |
| 1 | Pt-APA@Fe3O4/GO | DMF | - | reflux | 1 | 74 | [48] |
| 2 | Cu(II) PNP pincer complexes | CH3CN | - | r.t. | 16 | 69 | [49] |
| 3 | PdCl2 | Toluene | - | 100 | 8 | 99 | [50] |
| 4 | Pd NPs | Euphorbia granulate leaf extract | - | r.t. | 5 | 92 | [56] |
| 5 | Pd/C | H2O/EtOH | - | r.t. | 24 | 96 | [51] |
| 6 | PICB-NHC@Pd | EtOH | - | 80 | 36 | 89 | [52] |
| 7 | Polyimide@Pd | H2O | TBAB | 60 | 1 | 95 | [53] |
| 8 | CS@Pd | - | MW | r.t. | 0.1 | 99 | [54] |
| 9 | CS/starch @Pd | toluene | - | 50 | 0.1 | 98 | [55] |
| 10 | CS/PVA/MMT @Pd | H2O/EtOH | - | 80 | 12 | 97 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Z.; Wang, Z.; Li, Y.; Li, W.; Zheng, K.; Xiao, Z.; Wang, W.; Caiqin, Q. Preparation of a Montmorillonite-Modified Chitosan Film-Loaded Palladium Heterogeneous Catalyst and its Application in the Preparation of Biphenyl Compounds. Molecules 2022, 27, 8984. https://doi.org/10.3390/molecules27248984
Meng Z, Wang Z, Li Y, Li W, Zheng K, Xiao Z, Wang W, Caiqin Q. Preparation of a Montmorillonite-Modified Chitosan Film-Loaded Palladium Heterogeneous Catalyst and its Application in the Preparation of Biphenyl Compounds. Molecules. 2022; 27(24):8984. https://doi.org/10.3390/molecules27248984
Chicago/Turabian StyleMeng, Zhifei, Zijian Wang, Yu Li, Wei Li, Kewang Zheng, Zufeng Xiao, Wei Wang, and Qin Caiqin. 2022. "Preparation of a Montmorillonite-Modified Chitosan Film-Loaded Palladium Heterogeneous Catalyst and its Application in the Preparation of Biphenyl Compounds" Molecules 27, no. 24: 8984. https://doi.org/10.3390/molecules27248984
APA StyleMeng, Z., Wang, Z., Li, Y., Li, W., Zheng, K., Xiao, Z., Wang, W., & Caiqin, Q. (2022). Preparation of a Montmorillonite-Modified Chitosan Film-Loaded Palladium Heterogeneous Catalyst and its Application in the Preparation of Biphenyl Compounds. Molecules, 27(24), 8984. https://doi.org/10.3390/molecules27248984