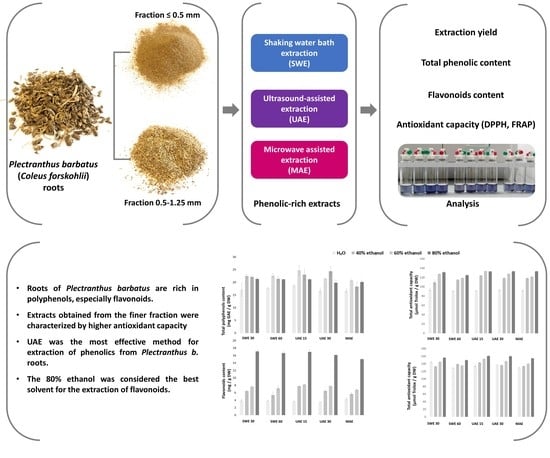
Bioactive Properties of Extracts from Plectranthus barbatus (Coleus forskohlii) Roots Received Using Various Extraction Methods
Abstract
:1. Introduction
2. Results and Discussion
3. Material and Methods
3.1. Plant Material and Chemicals
3.2. Plectranthus Barbatus (Coleus forskohlii) Root Extraction
3.3. Determination of the Extraction Yield
3.4. Determination of Total Antioxidant Capacity
3.5. Determination of Total Polyphenolic Content
3.6. Determination of Flavonoids Content
3.7. UHPLC–DAD–ESI–MS/MS Analysis of Phenolic Compounds
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lukhoba, C.W.; Simmonds, M.S.J.; Paton, A.J. Plectranthus: A review of ethnobotanical uses. J. Ethnopharmacol. 2006, 103, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Alasbahi, R.H.; Melzig, M.F. Plectranthus barbatus: A review of phytochemistry, ethnobotanical uses and pharmacology—Part 1. Planta Med. 2010, 76, 653–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavitha, C.; Rajamani, K.; Vadivel, E. Coleus forskohlii: A comprehensive review on morphology, phytochemistry and pharmacological aspects. J. Med. Plants Res. 2010, 4, 278–285. [Google Scholar]
- Rice, L.J.; Brits, G.J.; Potgieter, C.J.; Van Staden, J. Plectranthus: A plant for the future? S. Afr. J. Bot. 2011, 77, 947–959. [Google Scholar] [CrossRef] [Green Version]
- Chew, C.S. Forskolin stimulation of acid and pepsinogen secretion in isolated gastric glands. AJP-Cell Physiol. 1983, 245, C371–C380. [Google Scholar] [CrossRef]
- Lakshmanan, G.M.A.; Manikandan, S. Review on pharmacological effects of Plectranthus forskohlii (Willd) Briq. Int. Lett. Nat. Sci. 2015, 28, 1–9. [Google Scholar]
- Seamon, K.B.; Padgett, W.; Daly, J.W. Forskolin: Unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc. Natl. Acad. Sci. USA 1981, 78, 3363–3367. [Google Scholar] [CrossRef] [Green Version]
- Seamon, K.B.; Daly, J.W.; Metzger, H.; De Souza, N.J.; Reden, J. Structure-activity relationships for activation of adenylate cyclase by the diterpene forskolin and its derivatives. J. Med. Chem. 1983, 26, 436–439. [Google Scholar] [CrossRef]
- Daly, J.W. Forskolin, adenylate cyclase, and cell physiology: An overview. Adv. Cycl. Nucleotide Protein Phosphorylation Res. 1984, 17, 81–89. [Google Scholar]
- Alasbahi, R.H.; Melzig, M.F. Plectranthus barbatus: A review of phytochemistry, ethnobotanical uses and pharmacology—Part 2. Planta Med. 2010, 76, 753–765. [Google Scholar] [CrossRef]
- Kanne, H.; Burte, N.P.; Prasanna, V.; Gujjula, R. Extraction and elemental analysis of Coleus forskohlii extract. Pharmacogn. Res. 2015, 7, 237–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, C.; Papa, D.; Hübner, M.; Mou, T.C.; Lushington, G.H.; Seifert, R. Activation and inhibition of adenylyl cyclase isoforms by forskolin analogs. J. Pharmacol. Exp. Ther. 2008, 325, 27–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, S.; Mok, W.; Phaneuf, S. Forskolin inhibits platelet-activating factor binding to platelet receptors independently of adenylyl cyclase activation. Eur. J. Pharmacol. 1993, 245, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Dubey, M.P.; Srimal, R.C.; Nityanand, S.; Dhawan, B.N. Pharmacological studies on coleonol, a hypotensive diterpene from Coleus forskohlii. J. Ethnopharmacol. 1981, 3, 1–13. [Google Scholar] [CrossRef]
- Jagtap, M.; Chandola, H.M.; Ravishankar, B. Clinical efficacy of Coleus forskohlii (Willd.) Briq. (Makandi) in hypertension of geriatric population. AYU 2011, 32, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, J.; Sears, M.; Bausher, L. Forskolin lowers intraocular pressure by reducing aqueous inflow. Investig. Ophthalmol. Vis. Sci. 1984, 25, 268–277. [Google Scholar]
- Ho, R.; Shi, Q.H. Forskolin as a novel lipolytic agent. Biochem. Biophys. Res. Commun. 1982, 107, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Litosch, I.; Hudson, T.H.; Mills, I.; Li, S.Y.; Fain, J.N. Forskolin as an activator of cyclic AMP accumulation and lipolysis in rat adipocytes. Mol. Pharmacol. 1982, 22, 109–115. [Google Scholar]
- Okuda, H.; Morimoto, C.; Tsujita, T. Relationship between cyclic AMP production and lipolysis induced by forskolin in rat fat cells. J. Lipid Res. 1992, 2, 225–231. [Google Scholar] [CrossRef]
- Han, L.K.; Morimoto, C.; Yu, R.H.; Okuda, H. Effects of Coleus forskohlii on fat storage in ovariectomized rats. Yakugaku Zasshi 2005, 125, 449–453. [Google Scholar] [CrossRef] [Green Version]
- Henderson, S.; Magu, B.; Rasmussen, C.; Lancaster, S.; Kerksick, C.; Smith, P.; Melton, C.; Cowan, P.; Greenwood, M.; Earnest, C.; et al. Effects of Coleus forskohlii supplementation on body composition and hematological profiles in mildly overweight women. J. Int. Soc. Sports Nutr. 2005, 2, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Godard, M.P.; Johnson, B.A.; Richmond, S.R. Body composition and hormonal adaptations associated with forskolin consumption in overweight and obese men. Obes. Res. 2005, 8, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Marone, G.; Columbo, M.; Triggiani, M.; Cirillo, R.; Genovese, A.; Formisano, S. Inhibition of IgE-mediated release of histamine and peptide leukotriene from human basophils and mast cells by forskolin. Biochem. Pharmacol. 1987, 36, 13–20. [Google Scholar] [CrossRef] [PubMed]
- González-Sánchez, R.; Trujillo, X.; Trujillo-Hernández, B.; Vásquez, C.; Huerta, M.; Elizalde, A. Forskolin versus sodium cromoglycate for prevention of asthma attacks: A single-blinded clinical trial. J. Int. Med. Res. 2006, 34, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Huerta, M.; Urzúa, Z.; Trujillo, X.; González-Sánchez, R.; Trujillo-Hernández, B. Forskolin compared with beclomethasone for prevention of asthma attacks: A single-blind clinical trial. J. Int. Med. Res. 2010, 38, 661–668. [Google Scholar] [CrossRef]
- Al Musayeib, N.M.; Amina, M.; Al-Hamoud, G.A.; Mohamed, G.A.; Ibrahim, S.R.M.; Shabana, S. Plectrabarbene, a new abietane diterpene from Plectranthus barbatus aerial parts. Molecules 2020, 25, 2365. [Google Scholar] [CrossRef]
- Falé, P.L.; Madeira, P.J.; Florêncio, M.H.; Ascensão, L.; Serralheiro, M.L. Function of Plectranthus barbatus herbal tea as neuronal acetylcholinesterase inhibitor. Food Funct. 2011, 2, 130–136. [Google Scholar] [CrossRef]
- Borges, A.S.; Minozzo, B.R.; Santos, H.; Ardisson, J.S.; Rodrigues, R.P.; Romão, W.; Borges, W.; Gonçalves, R.; Beltrame, F.L.; Kitagawa, R.R. Plectranthus barbatus Andrews as anti-Helicobacter pylori agent with activity against adenocarcinoma gastric cells. Ind. Crops. Prod. 2020, 146, 112207. [Google Scholar] [CrossRef]
- Majeed, M.; Prakash, S. Composition and Methods Containing an Antimicrobial Essential Oil Extended from Coleus forskohlii. U.S. Patent No. 6,607,712 B2, 2003. [Google Scholar]
- Chatterjee, B.; Vittal, R.R. Quorum sensing modulatory and biofilm inhibitory activity of Plectranthus barbatus essential oil: A novel intervention strategy. Arch. Microbiol. 2021, 203, 1767–1778. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Arafa, N.M.; Aly, U.I. Antioxidant activity, phenol and flavonoid contents of plant and callus cultures of Plectranthus barbatus andrews. Egypt. Pharm. J. 2018, 17, 32–39. [Google Scholar] [CrossRef]
- Amina, M.; Al-Musayeib, N.M.; Alam, P.; Fadilah, S.; Aleanizy, F.S.; Alqahtni, F.Y.; Mansour, S.; Al-Said, M.S.; Al-Rashidi, N.S.; Shakeel, F. Cytotoxic evaluation and concurrent analysis of two diterpenes in the chloroform extract of Plectranthus barbatus using a validated HPTLC-UV method. Bull. Chem. Soc. Ethiop. 2018, 32, 407–419. [Google Scholar] [CrossRef]
- Amina, M.; Al-Musayeib, N.M.; Al-Said, M.S.; Rania, A.; Al-Zahrani, R.A.; Ibrahim, S.R.M.; Mohamed, G.A. Barbaterpene and barbatusterol, new constituents from Plectranthus barbatus growing in Saudi Arabia. Lett. Drug Des. Discov. 2018, 8, 851–856. [Google Scholar] [CrossRef]
- Kapewangolo, P.; Hussein, A.A.; Meyer, D. Inhibition of HIV-1 enzymes, antioxidant and anti-inflammatory activities of Plectranthus barbatus. J. Ethnopharmacol. 2013, 1, 184–190. [Google Scholar] [CrossRef] [Green Version]
- Kapewangolo, P.; Meyer, D. Plectranthus barbatus: Antioxidant, and other inhibitory responses against HIV/AIDS. In HIV/AIDS: Oxidative Stress and Dietary Antioxidants; Preedy, V.R., Watson, R.R., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 149–159. ISBN 9780128098530. [Google Scholar]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Wu, J.; Lin, L.; Chau, F.T. Ultrasound-assisted extraction of ginseng saponins from ginseng roots and cultured ginseng cells. Ultrason. Sonochem. 2001, 8, 347–352. [Google Scholar] [CrossRef]
- Yang, L.; Cao, Y.L.; Jiang, J.G.; Lin, Q.S.; Chen, J.; Zhu, L. Response surface optimization of ultrasound-assisted flavonoids extraction from the flower of Citrus aurantium L. var. amara Engl. J. Sep. Sci. 2010, 33, 1349–1355. [Google Scholar] [CrossRef]
- Tabaraki, R.; Heidarizadi, E.; Benvidi, A. Optimization of ultrasonic-assisted extraction of pomegranate (Punica granatum L.) peel antioxidants by response surface methodology. Sep. Purif. Technol. 2012, 98, 16–23. [Google Scholar] [CrossRef]
- Upadhyay, R.; Nachiappan, G.; Mishra, H.N. Ultrasound-assisted extraction of flavonoids and phenolic compounds from Ocimum tenuiflorum leaves. Food Sci. Biotechnol. 2015, 24, 1951–1958. [Google Scholar] [CrossRef]
- M’hiri, N.; Ioannou, I.; Boudhrioua, N.; Ghoul, M. Effect of different operating conditions on the extraction of phenolic compounds in orange peel. Food Bioprod. Process. 2015, 6, 161–170. [Google Scholar] [CrossRef]
- Uzel, R.A. Microwave-assisted green extraction technology for sustainable food processing: Emerging microwave technologies in industrial, agricultural, medical and food processing. In Emerging Microwave Technologies in Industrial, Agricultural, Medical and Food Processing; You, K.Y., Ed.; Intech Open: London, UK, 2018; pp. 159–178. [Google Scholar]
- Duan, W.; Jin, S.; Zhao, G.; Sun, P. Microwave-assisted extraction of anthocyanin from Chinese bayberry and its effects on anthocyanin stability. Food Sci. Technol. 2015, 35, 524–530. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, Y.; Wu, D.; Xu, M.; Chen, J. Microwave-assisted extraction of polyphenols from Camellia oleifera fruit hull. Molecules 2011, 16, 4428–4437. [Google Scholar] [CrossRef] [Green Version]
- Cardoso-Ugarte, G.A.; Juárez-Becerra, G.P.; Sosa-Morales, M.E.; López-Malo, A. Microwave-assisted extraction of essential oils from herbs. J. Microwave Power Electromagn. Energy 2013, 47, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Saifullah, M.; McCullum, R.; McCluskey, A.; Vuong, Q. Comparison of conventional extraction technique with ultrasound assisted extraction on recovery of phenolic compounds from lemon scented tea tree (Leptospermum petersonii) leaves. Heliyon 2020, 6, e03666. [Google Scholar] [CrossRef] [PubMed]
- Palsikowski, P.A.; Besen, L.M.; Klein, E.J.; Silva, C.; Silva, E.A. Optimization of ultrasound-assisted extraction of bioactive compounds from B. forficata subsp. Pruinosa. Can. J. Chem. Eng. 2020, 98, 2214–2226. [Google Scholar] [CrossRef]
- Ez Zoubi, Y.; Fadil, M.; Bousta, D.; El Ouali Lalami, A.; Lachkar, M.; Farah, A. Ultrasound-assisted extraction of phenolic compounds from Moroccan Lavandula stoechas L.: Optimization using response surface methodology. J. Chem. 2021, 2021, 8830902. [Google Scholar] [CrossRef]
- Jovanović, A.; Đorđević, V.; Zdunić, G.; Pljevljakušić, D.; Šavikin, K.; Gođevac, D.; Bugarski, B. Optimization of the extraction process of polyphenols from Thymus serpyllum L. herb using maceration, heat- and ultrasound-assisted techniques. Sep. Purif. Technol. 2017, 179, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Patra, M.; Salonen, E.; Terama, E.; Vattulainen, I.; Faller, R.; Lee, B.W.; Holopainen, J.; Karttunen, M. Under the influence of alcohol: The effect of ethanol and methanol on lipid bilayers. Biophys. J. 2006, 90, 1121–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen-Kim, M.T.; Truong, Q.C.; Nguyen, M.T.; Cao-Thi, B.H.; Tong, T.D.; Dao, T.P.; Tran, T.H.; Van Tan, L.; Le, X.T. Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity. Open Chem. 2021, 19, 1043–1051. [Google Scholar] [CrossRef]
- Atulkar, P.; Thakur, R.; Singh, P. Preliminary phytochemical analysis of root extracts of Coleus forskohlii briq. Int. J. of Adv. Res. 2015, 3, 1145–1150. [Google Scholar]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Eisner, P. How does the phenol structure influence the results of the Folin-Ciocalteu assay? Antioxidants 2021, 10, 811. [Google Scholar] [CrossRef]
- Taher, M.; El-Daly, N.; El-Khateeb, A.; Hassan, S.; Elsherbiny, E. Chemical Composition, Antioxidant, Antitumor and Antifungal Activities of Methanolic Extracts of Coleus blumei, Plectranthus amboinicus and Salvia splendens (Lamiaceae). J. Agric. Chem. Biotechnol. 2021, 12, 177–187. [Google Scholar] [CrossRef]
- Benedec, D.; Hanganu, D.; Oniga, I.; Tiperciuc, B.; Olah, N.K.; Raita, O.; Bischin, C.; Silaghi-Dumitrescu, R.; Vlase, L. Assessment of rosmarinic acid content in six Lamiaceae species extracts and their antioxidant and antimicrobial potential. Pak. J. Pharm. Sci. 2015, 28, 2297–2303. [Google Scholar]
- Oracz, J.; Żyżelewicz, D. In Vitro Antioxidant Activity and FTIR Characterization of High-Molecular Weight Melanoidin Fractions from Different Types of Cocoa Beans. Antioxidants 2019, 8, 560. [Google Scholar] [CrossRef] [Green Version]
- Georgiadou, E.C.; Kowalska, E.; Patla, K.; Kulbat, K.; Smolińska, B.; Leszczyńska, J.; Fotopoulos, V. Influence of heavy metals (Ni, Cu, and Zn) on nitro-oxidative stress responses, proteome regulation and allergen production in Basil (Ocimum basilicum L.) plants. Front. Plant Sci. 2018, 9, 862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singelton, V.L.; Rossi, J.A. Colorymetry of total phenolics with phosphomolybdic phodphotungstics acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Oracz, J.; Żyżelewicz, D.; Pacholczyk-Sienicka, B. UHPLC-DAD-ESI-HRMS/MS profile of phenolic compounds in northern red oak (Quercus rubra L., syn. Q. borealis F. Michx) seeds and its transformation during thermal processing. Ind. Crops Prod. 2022, 189, 115860. [Google Scholar]
- Pudziuvelyte, L.; Liaudanskas, M.; Jekabsone, A.; Sadauskiene, I.; Bernatoniene, J. Elsholtzia ciliata (Thunb.) Hyl. Extracts from Different Plant Parts: Phenolic Composition, Antioxidant, and Anti-Inflammatory Activities. Molecules 2020, 25, 1153. [Google Scholar] [CrossRef] [PubMed]








| Fraction | Extraction Method | Solvent | Extraction Yield (%) |
|---|---|---|---|
| Fraction ≤ 0.5 mm | SWE 30 | H2O | 29.6 ± 0.7 a |
| 40% ethanol | 32.7 ± 0.7 c | ||
| 60% ethanol | 30.9 ± 0.5 b | ||
| 80% ethanol | 28.7 ± 0.5 a | ||
| SWE 60 | H2O | 32.7 ± 0.9 b | |
| 40% ethanol | 32.2 ± 0.7 b | ||
| 60% ethanol | 33.9 ± 0.5 c | ||
| 80% ethanol | 28.4 ± 0.6 a | ||
| UAE 15 | H2O | 32.2 ± 0.9 c | |
| 40% ethanol | 29.3 ± 0.6 a,b | ||
| 60% ethanol | 30.0 ± 0.3 b | ||
| 80% ethanol | 28.3 ± 0.5 a | ||
| UAE 30 | H2O | 31.2 ± 0.9 b | |
| 40% ethanol | 32.9 ± 0.8 c | ||
| 60% ethanol | 31.4 ± 0.3 b | ||
| 80% ethanol | 28.7 ± 0.7 a | ||
| MAE | H2O | 33.9 ± 0.9 c | |
| 40% ethanol | 33.6 ± 0.3 c | ||
| 60% ethanol | 32.5 ± 0.7 b,c | ||
| 80% ethanol | 29.2 ± 0.7 a | ||
| Fraction 0.5–1.25 mm | SWE 30 | H2O | 31.9 ± 0.9 c |
| 40% ethanol | 34.2 ± 0.6 d | ||
| 60% ethanol | 30.1 ± 0.8 b | ||
| 80% ethanol | 27.3 ± 0.9 a | ||
| SWE 60 | H2O | 31.5 ± 0.8 b | |
| 40% ethanol | 31.4 ± 0.7 b | ||
| 60% ethanol | 31.9 ± 0.1 b | ||
| 80% ethanol | 27.7 ± 0.3 a | ||
| UAE 15 | H2O | 35.1 ± 0.8 c | |
| 40% ethanol | 32.4 ± 0.7 b | ||
| 60% ethanol | 31.4 ± 0.6 b | ||
| 80% ethanol | 27.1 ± 0.3 a | ||
| UAE 30 | H2O | 33.0 ± 0.6 c | |
| 40% ethanol | 32.0 ± 0.5 c | ||
| 60% ethanol | 30.5 ± 0.9 b | ||
| 80% ethanol | 27.2 ± 0.8 a | ||
| MAE | H2O | 36.4 ± 0.8 d | |
| 40% ethanol | 32.0 ± 0.5 c | ||
| 60% ethanol | 30.1 ± 0.7 b | ||
| 80% ethanol | 24.4 ± 0.8 a |
| Extraction Method | Solvent | QA | PA | 4-HBA | PAL | GA | HBA-hex | VA | CA | SA | p-CA | FA | 2-HBA | EA | HGA | RA | TOTAL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SWE 30 Fraction <0.5 | H2O | 0.259 ± 0.032 | 0.376 ± 0.083 | 0.122 ± 0.017 | 0.073 ± 0.016 | 0.067 ± 0.014 | 0.015 ± 0.000 | 0.041 ± 0.000 | 0.237 ± 0.035 | 0.008 ± 0.000 | 0.008 ± 0.003 | 0.018 ± 0.000 | 0.025 ± 0.000 | nd | 0.035 ± 0.001 | 0.153 ± 0.007 | 1.437 ± 0.065 |
| 40% EtOH | 0.466 ± 0.113 | 0.418 ± 0.025 | 0.230 ± 0.025 | 0.123 ± 0.023 | 0.127 ± 0.036 | 0.027 ± 0.001 | 0.054 ± 0.000 | 0.169 ± 0.025 | 0.011 ± 0.001 | 0.008 ± 0.001 | 0.021 ± 0.000 | nd | 0.047 ± 0.003 | 0.148 ± 0.012 | 1.113 ± 0.028 | 2.962 ± 0.098 | |
| 60% EtOH | 0.405 ± 0.061 | 0.428 ± 0.023 | 0.238 ± 0.035 | 0.122 ± 0.013 | 0.131 ± 0.013 | 0.027 ± 0.002 | 0.056 ± 0.001 | 0.176 ± 0.026 | 0.015 ± 0.003 | 0.008 ± 0.001 | 0.022 ± 0.000 | 0.025 ± 0.000 | 0.073 ± 0.001 | 0.157 ± 0.012 | 1.179 ± 0.021 | 3.062 ± 0.078 | |
| 80% EtOH | 0.379 ± 0.033 | 0.401 ± 0.095 | 0.229 ± 0.016 | 0.124 ± 0.046 | 0.135 ± 0.026 | 0.026 ± 0.002 | 0.057 ± 0.002 | 0.174 ± 0.023 | 0.015 ± 0.004 | 0.008 ± 0.002 | 0.023 ± 0.001 | 0.022 ± 0.001 | 0.045 ± 0.002 | 0.145 ± 0.021 | 1.151 ± 0.011 | 2.934 ± 0.093 | |
| SWE 30 Fraction 0.5–1.25 | H2O | 0.415 ± 0.014 | 0.435 ± 0.083 | 0.154 ± 0.012 | 0.112 ± 0.013 | 0.104 ± 0.013 | 0.024 ± 0.001 | 0.057 ± 0.010 | 0.244 ± 0.035 | 0.011 ± 0.000 | 0.009 ± 0.001 | 0.018 ± 0.003 | 0.019 ± 0.001 | 0.047 ± 0.002 | 0.037 ± 0.009 | 0.214 ± 0.003 | 1.900 ± 0.057 |
| 40% EtOH | 0.499 ± 0.021 | 0.374 ± 0.123 | 0.213 ± 0.034 | 0.129 ± 0.024 | 0.128 ± 0.023 | 0.031 ± 0.005 | 0.058 ± 0.000 | 0.161 ± 0.032 | 0.011 ± 0.002 | 0.008 ± 0.003 | 0.020 ± 0.004 | 0.025 ± 0.000 | 0.036 ± 0.001 | 0.148 ± 0.008 | 1.126 ± 0.022 | 2.968 ± 0.062 | |
| 60% EtOH | 0.523 ± 0.032 | 0.374 ± 0.065 | 0.238 ± 0.076 | 0.136 ± 0.037 | 0.135 ± 0.015 | 0.033 ± 0.000 | 0.065 ± 0.010 | 0.176 ± 0.016 | 0.012 ± 0.003 | 0.009 ± 0.000 | 0.022 ± 0.003 | 0.023 ± 0.002 | 0.038 ± 0.000 | 0.178 ± 0.016 | 1.196 ± 0.004 | 3.157 ± 0.060 | |
| 80% EtOH | 0.548 ± 0.112 | 0.322 ± 0.023 | 0.200 ± 0.023 | 0.123 ± 0.013 | 0.128 ± 0.012 | 0.025 ± 0.000 | 0.058 ± 0.000 | 0.162 ± 0.012 | 0.011 ± 0.000 | 0.008 ± 0.000 | 0.021 ± 0.005 | 0.020 ± 0.002 | 0.033 ± 0.000 | 0.163 ± 0.024 | 1.066 ± 0.024 | 2.888 ± 0.078 | |
| SWE 60 Fraction <0.5 | H2O | 0.278 ± 0.033 | 0.425 ± 0.035 | 0.138 ± 0.044 | 0.086 ± 0.012 | 0.078 ± 0.019 | 0.023 ± 0.000 | 0.050 ± 0.002 | 0.286 ± 0.033 | 0.008 ± 0.000 | 0.009 ± 0.000 | 0.021 ± 0.002 | 0.019 ± 0.000 | 0.134 ± 0.000 | 0.043 ± 0.006 | 0.179 ± 0.021 | 1.777 ± 0.062 |
| 40% EtOH | 0.373 ± 0.024 | 0.412 ± 0.038 | 0.230 ± 0.016 | 0.125 ± 0.011 | 0.126 ± 0.028 | 0.036 ± 0.001 | 0.054 ± 0.008 | 0.169 ± 0.017 | 0.010 ± 0.000 | 0.008 ± 0.000 | 0.021 ± 0.000 | 0.023 ± 0.000 | 0.074 ± 0.012 | 0.143 ± 0.010 | 1.106 ± 0.034 | 2.910 ± 0.069 | |
| 60% EtOH | 0.420 ± 0.125 | 0.444 ± 0.054 | 0.239 ± 0.012 | 0.129 ± 0.033 | 0.141 ± 0.015 | 0.030 ± 0.000 | 0.060 ± 0.007 | 0.192 ± 0.011 | 0.016 ± 0.004 | 0.008 ± 0.000 | 0.024 ± 0.005 | 0.035 ± 0.000 | 0.085 ± 0.014 | 0.169 ± 0.009 | 1.264 ± 0.072 | 3.256 ± 0.098 | |
| 80% EtOH | 0.411 ± 0.052 | 0.441 ± 0.066 | 0.246 ± 0.049 | 0.134 ± 0.086 | 0.146 ± 0.023 | 0.025 ± 0.000 | 0.062 ± 0.001 | 0.194 ± 0.049 | 0.017 ± 0.005 | 0.0090 ± 0.001 | 0.024 ± 0.006 | 0.024 ± 0.001 | 0.054 ± 0.002 | 0.165 ± 0.027 | 1.242 ± 0.086 | 3.194 ± 0.083 | |
| SWE 60 Fraction 0.5–1.25 | H2O | 0.426 ± 0.093 | 0.435 ± 0.15 | 0.158 ± 0.010 | 0.125 ± 0.013 | 0.108 ± 0.047 | 0.033 ± 0.002 | 0.060 ± 0.000 | 0.221 ± 0.054 | 0.013 ± 0.001 | 0.049 ± 0.002 | 0.019 ± 0.000 | 0.019 ± 0.000 | 0.047 ± 0.000 | 0.029 ± 0.005 | 0.130 ± 0.012 | 1.873 ± 0.068 |
| 40% EtOH | 0.980 ± 0.123 | 0.353 ± 0.065 | 0.207 ± 0.084 | 0.122 ± 0.048 | 0.121 ± 0.014 | 0.029 ± 0.003 | 0.057 ± 0.000 | 0.155 ± 0.030 | 0.011 ± 0.001 | 0.007 ± 0.000 | 0.018 ± 0.001 | nd | 0.027 ± 0.000 | 0.153 ± 0.014 | 1.145 ± 0.045 | 3.386 ± 0.084 | |
| 60% EtOH | 0.505 ± 0.032 | 0.336 ± 0.023 | 0.226 ± 0.056 | 0.131 ± 0.016 | 0.133 ± 0.006 | 0.056 ± 0.002 | 0.063 ± 0.004 | 0.165 ± 0.014 | 0.018 ± 0.002 | 0.009 ± 0.000 | 0.020 ± 0.001 | 0.021 ± 0.000 | 0.033 ± 0.000 | 0.178 ± 0.014 | 1.065 ± 0.090 | 2.959 ± 0.079 | |
| 80% EtOH | 0.543 ± 0.071 | 0.328 ± 0.023 | 0.196 ± 0.034 | 0.117 ± 0.013 | 0.128 ± 0.013 | 0.022 ± 0.001 | 0.058 ± 0.000 | 0.161 ± 0.035 | 0.011 ± 0.004 | 0.008 ± 0.000 | 0.019 ± 0.000 | 0.018 ± 0.000 | 0.031 ± 0.000 | 0.159 ± 0.032 | 1.120 ± 0.128 | 2.919 ± 0.067 | |
| UAE 15 Fraction <0.5 | H2O | 0.353 ± 0.094 | 0.543 ± 0.093 | 0.167 ± 0.013 | 0.103 ± 0.044 | 0.094 ± 0.014 | 0.031 ± 0.000 | 0.059 ± 0.000 | 0.317 ± 0.032 | 0.014 ± 0.001 | 0.0114 ± 0.002 | 0.025 ± 0.001 | 0.027 ± 0.001 | 0.097 ± 0.001 | 0.055 ± 0.011 | 0.191 ± 0.033 | 2.087 ± 0.063 |
| 40% EtOH | 0.503 ± 0.035 | 0.445 ± 0.034 | 0.251 ± 0.030 | 0.153 ± 0.013 | 0.170 ± 0.024 | 0.029 ± 0.000 | 0.057 ± 0.007 | 0.179 ± 0.002 | 0.015 ± 0.002 | 0.0082 ± 0.000 | 0.024 ± 0.000 | 0.025 ± 0.002 | 0.125 ± 0.021 | 0.168 ± 0.012 | 1.214 ± 0.256 | 3.366 ± 0.075 | |
| 60% EtOH | 0.414 ± 0.153 | 0.431 ± 0.162 | 0.240 ± 0.084 | 0.125 ± 0.015 | 0.135 ± 0.046 | 0.026 ± 0.000 | 0.057 ± 0.018 | 0.180 ± 0.012 | 0.017 ± 0.004 | 0.009 ± 0.000 | 0.022 ± 0.003 | 0.024 ± 0.000 | 0.114 ± 0.031 | 0.168 ± 0.013 | 1.186 ± 0.313 | 3.148 ± 0.078 | |
| 80% EtOH | 0.476 ± 0.049 | 0.392 ± 0.036 | 0.219 ± 0.016 | 0.119 ± 0.013 | 0.132 ± 0.011 | 0.022 ± 0.000 | 0.055 ± 0.017 | 0.172 ± 0.014 | 0.014 ± 0.001 | 0.008 ± 0.000 | 0.022 ± 0.000 | 0.021 ± 0.001 | 0.044 ± 0.001 | 0.142 ± 0.035 | 1.101 ± 0.084 | 2.939 ± 0.063 | |
| UAE 15 Fraction 0.5–1.25 | H2O | 0.473 ± 0.176 | 0.480 ± 0.122 | 0.159 ± 0.049 | 0.129 ± 0.048 | 0.112 ± 0.011 | 0.033 ± 0.000 | 0.058 ± 0.007 | 0.240 ± 0.024 | 0.012 ± 0.002 | 0.010 ± 0.000 | 0.019 ± 0.000 | 0.024 ± 0.002 | 0.052 ± 0.001 | 0.040 ± 0.015 | 0.219 ± 0.025 | 2.060 ± 0.065 |
| 40% EtOH | 0.469 ± 0.0951 | 0.350 ± 0.064 | 0.207 ± 0.097 | 0.131 ± 0.029 | 0.124 ± 0.012 | 0.027 ± 0.000 | 0.058 ± 0.019 | 0.149 ± 0.014 | 0.011 ± 0.000 | 0.008 ± 0.000 | 0.019 ± 0.001 | 0.021 ± 0.000 | 0.060 ± 0.002 | 0.149 ± 0.015 | 1.064 ± 0.073 | 2.847 ± 0.069 | |
| 60% EtOH | 0.513 ± 0.0451 | 0.379 ± 0.074 | 0.197 ± 0.087 | 0.135 ± 0.017 | 0.129 ± 0.021 | 0.024 ± 0.000 | 0.057 ± 0.009 | 0.165 ± 0.011 | 0.012 ± 0.006 | 0.008 ± 0.001 | 0.021 ± 0.000 | 0.022 ± 0.000 | 0.035 ± 0.001 | 0.167 ± 0.029 | 1.181 ± 0.163 | 3.045 ± 0.075 | |
| 80% EtOH | 0.457 ± 0.133 | 0.322 ± 0.082 | 0.192 ± 0.028 | 0.124 ± 0.025 | 0.124 ± 0.009 | 0.045 ± 0.001 | 0.055 ± 0.000 | 0.158 ± 0.021 | 0.016 ± 0.007 | 0.008 ± 0.000 | 0.019 ± 0.001 | nd | 0.030 ± 0.00 | 0.136 ± 0.089 | 0.969 ± 0.033 | 2.655 ± 0.085 | |
| UAE 30 Fraction <0.5 | H2O | 0.275 ± 0.021 | 0.398 ± 0.045 | 0.135 ± 0.015 | 0.078 ± 0.017 | 0.074 ± 0.012 | 0.018 ± 0.002 | 0.044 ± 0.004 | 0.233 ± 0.026 | 0.008 ± 0.006 | 0.0085 ± 0.000 | 0.018 ± 0.002 | 0.016 ± 0.000 | 0.065 ± 0.002 | 0.041 ± 0.003 | 0.202 ± 0.025 | 1.614 ± 0.092 |
| 40% EtOH | 0.393 ± 0.064 | 0.409 ± 0.085 | 0.212 ± 0.049 | 0.118 ± 0.015 | 0.124 ± 0.012 | 0.025 ± 0.000 | 0.054 ± 0.0014 | 0.169 ± 0.016 | 0.010 ± 0.001 | 0.0076 ± 0.000 | 0.022 ± 0.001 | 0.023 ± 0.000 | 0.078 ± 0.004 | 0.148 ± 0.013 | 1.104 ± 0.085 | 2.897 ± 0.078 | |
| 60% EtOH | 0.396 ± 0.063 | 0.417 ± 0.075 | 0.223 ± 0.026 | 0.122 ± 0.024 | 0.124 ± 0.002 | 0.046 ± 0.0130 | 0.055 ± 0.120 | 0.174 ± 0.024 | 0.014 ± 0.002 | 0.008 ± 0.002 | 0.022 ± 0.000 | nd | 0.079 ± 0.000 | 0.152 ± 0.064 | 1.108 ± 0.091 | 2.940 ± 0.072 | |
| 80% EtOH | 0.381 ± 0.047 | 0.402 ± 0.042 | 0.227 ± 0.012 | 0.120 ± 0.046 | 0.131 ± 0.012 | 0.017 ± 0.000 | 0.056 ± 0.011 | 0.179 ± 0.079 | 0.017 ± 0.03 | 0.008 ± 0.000 | 0.022 ± 0.000 | 0.021 ± 0.000 | 0.084 ± 0.000 | 0.150 ± 0.032 | 1.084 ± 0.049 | 2.900 ± 0.089 | |
| UAE 30 Fraction 0.5–1.25 | H2O | 0.402 ± 0.074 | 0.501 ± 0.062 | 0.164 ± 0.014 | 0.129 ± 0.025 | 0.114 ± 0.002 | 0.021 ± 0.000 | 0.059 ± 0.000 | 0.256 ± 0.085 | 0.012 ± 0.001 | 0.010 ± 0.003 | 0.020 ± 0.002 | 0.020 ± 0.000 | 0.084 ± 0.001 | 0.039 ± 0.010 | 0.219 ± 0.020 | 2.051 ± 0.084 |
| 40% EtOH | 0.451 ± 0.094 | 0.352 ± 0.034 | 0.202 ± 0.016 | 0.125 ± 0.035 | 0.120 ± 0.007 | 0.024 ± 0.001 | 0.054 ± 0.000 | 0.145 ± 0.033 | 0.011 ± 0.000 | 0.007 ± 0.000 | 0.019 ± 0.001 | nd | 0.028 ± 0.000 | 0.154 ± 0.031 | 1.038 ± 0.004 | 2.730 ± 0.069 | |
| 60% EtOH | 0.496 ± 0.117 | 0.350 ± 0.013 | 0.217 ± 0.014 | 0.129 ± 0.032 | 0.129 ± 0.031 | 0.022 ± 0.000 | 0.058 ± 0.007 | 0.163 ± 0.004 | 0.012 ± 0.000 | 0.009 ± 0.002 | 0.021 ± 0.002 | 0.021 ± 0.000 | 0.025 ± 0.000 | 0.161 ± 0.012 | 1.129 ± 0.144 | 2.942 ± 0.075 | |
| 80% EtOH | 0.566 ± 0.053 | 0.326 ± 0.063 | 0.203 ± 0.014 | 0.125 ± 0.021 | 0.126 ± 0.002 | 0.043 ± 0.000 | 0.058 ± 0.006 | 0.165 ± 0.048 | 0.012 ± 0.003 | 0.008 ± 0.000 | 0.022 ± 0.000 | 0.026 ± 0.002 | 0.026 ± 0.000 | 0.158 ± 0.087 | 1.126 ± 0.079 | 2.990 ± 0.082 | |
| MAE Fraction <0.5 | H2O | 0.278 ± 0.037 | 0.387 ± 0.014 | 0.132 ± 0.014 | 0.077 ± 0.037 | 0.075 ± 0.011 | 0.014 ± 0.000 | 0.044 ± 0.005 | 0.201 ± 0.024 | 0.011 ± 0.001 | 0.008 ± 0.000 | 0.017 ± 0.000 | 0.019 ± 0.000 | 0.069 ± 0.003 | 0.037 ± 0.009 | 0.195 ± 0.041 | 1.564 ± 0.085 |
| 40% EtOH | 0.414 ± 0.033 | 0.421 ± 0.093 | 0.216 ± 0.062 | 0.124 ± 0.871 | 0.125 ± 0.002 | 0.019 ± 0.002 | 0.052 ± 0.009 | 0.156 ± 0.053 | 0.015 ± 0.005 | 0.008 ± 0.001 | 0.021 ± 0.002 | nd | 0.067 ± 0.000 | 0.149 ± 0.013 | 1.072 ± 0.086 | 2.859 ± 0.089 | |
| 60% EtOH | 0.398 ± 0.063 | 0.395 ± 0.062 | 0.221 ± 0.032 | 0.123 ± 0.021 | 0.131 ± 0.037 | 0.018 ± 0.001 | 0.054 ± 0.008 | 0.169 ± 0.055 | 0.013 ± 0.002 | 0.008 ± 0.000 | 0.022 ± 0.000 | 0.023 ± 0.001 | 0.072 ± 0.002 | 0.155 ± 0.005 | 1.134 ± 0.099 | 2.936 ± 0.078 | |
| 80% EtOH | 0.485 ± 0.125 | 0.398 ± 0.072 | 0.218 ± 0.021 | 0.125 ± 0.0784 | 0.133 ± 0.002 | 0.033 ± 0.004 | 0.056 ± 0.002 | 0.175 ± 0.034 | 0.017 ± 0.000 | 0.009 ± 0.000 | 0.023 ± 0.000 | 0.018 ± 0.000 | 0.038 ± 0.000 | 0.145 ± 0.001 | 1.103 ± 0.069 | 2.976 ± 0.093 | |
| MAE Fraction 0.5–1.25 | H2O | 0.432 ± 0.043 | 0.468 ± 0.021 | 0.133 ± 0.051 | 0.122 ± 0.022 | 0.099 ± 0.009 | 0.016 ± 0.000 | 0.056 ± 0.001 | 0.218 ± 0.068 | 0.014 ± 0.004 | 0.007 ± 0.000 | 0.018 ± 0.000 | 0.023 ± 0.000 | 0.071 ± 0.002 | 0.031 ± 0.009 | 0.132 ± 0.071 | 1.841 ± 0.078 |
| 40% EtOH | 0.738 ± 0.025 | 0.352 ± 0.046 | 0.203 ± 0.016 | 0.129 ± 0.037 | 0.120 ± 0.002 | 0.044 ± 0.000 | 0.056 ± 0.005 | 0.147 ± 0.013 | 0.016 ± 0.000 | 0.008 ± 0.000 | 0.018 ± 0.000 | nd | 0.075 ± 0.005 | 0.146 ± 0.031 | 1.016 ± 0.131 | 3.068 ± 0.094 | |
| 60% EtOH | 0.470 ± 0.024 | 0.342 ± 0.068 | 0.198 ± 0.043 | 0.124 ± 0.0.029 | 0.127 ± 0.014 | 0.040 ± 0.000 | 0.057 ± 0.007 | 0.156 ± 0.031 | 0.012 ± 0.001 | 0.008 ± 0.000 | 0.019 ± 0.000 | 0.021 ± 0.000 | 0.024 ± 0.000 | 0.162 ± 0.008 | 1.078 ± 0.071 | 2.839 ± 0.077 | |
| 80% EtOH | 0.395 ± 0.085 | 0.262 ± 0.017 | 0.171 ± 0.034 | 0.108 ± 0.0.011 | 0.114 ± 0.014 | nd | 0.051 ± 0.000 | 0.136 ± 0.009 | 0.014 ± 0.000 | 0.007 ± 0.000 | 0.017 ± 0.000 | 0.012 ± 0.000 | 0.058 ± 0.009 | 0.125 ± 0.097 | 0.806 ± 0.032 | 2.277 ± 0.085 |
| Extraction Method | Solvent | Time of Extraction |
|---|---|---|
| Shaking water bath extraction (SWA) | H2O | 30 min |
| 40% ethanol | 30 min | |
| 60% ethanol | 30 min | |
| 80% ethanol | 30 min | |
| H2O | 60 min | |
| 40% ethanol | 60 min | |
| 60% ethanol | 60 min | |
| 80% ethanol | 60 min | |
| Ultrasound-assisted extraction (UAE) | H2O | 15 min |
| 40% ethanol | 15 min | |
| 60% ethanol | 15 min | |
| 80% ethanol | 15 min | |
| H2O | 30 min | |
| 40% ethanol | 30 min | |
| 60% ethanol | 30 min | |
| 80% ethanol | 30 min | |
| Microwave assisted extraction (MAE) | H2O | 10 s |
| 40% ethanol | 9 s | |
| 60% ethanol | 8 s | |
| 80% ethanol | 7 s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulbat-Warycha, K.; Oracz, J.; Żyżelewicz, D. Bioactive Properties of Extracts from Plectranthus barbatus (Coleus forskohlii) Roots Received Using Various Extraction Methods. Molecules 2022, 27, 8986. https://doi.org/10.3390/molecules27248986
Kulbat-Warycha K, Oracz J, Żyżelewicz D. Bioactive Properties of Extracts from Plectranthus barbatus (Coleus forskohlii) Roots Received Using Various Extraction Methods. Molecules. 2022; 27(24):8986. https://doi.org/10.3390/molecules27248986
Chicago/Turabian StyleKulbat-Warycha, Kamila, Joanna Oracz, and Dorota Żyżelewicz. 2022. "Bioactive Properties of Extracts from Plectranthus barbatus (Coleus forskohlii) Roots Received Using Various Extraction Methods" Molecules 27, no. 24: 8986. https://doi.org/10.3390/molecules27248986
APA StyleKulbat-Warycha, K., Oracz, J., & Żyżelewicz, D. (2022). Bioactive Properties of Extracts from Plectranthus barbatus (Coleus forskohlii) Roots Received Using Various Extraction Methods. Molecules, 27(24), 8986. https://doi.org/10.3390/molecules27248986