Structure and Population of Complex Ionic Species in FeCl2 Aqueous Solution by X-ray Absorption Spectroscopy
Abstract
:1. Introduction
2. Results
2.1. Fe K-Edge XANES Results
2.2. Fe K-Edge EXAFS Results
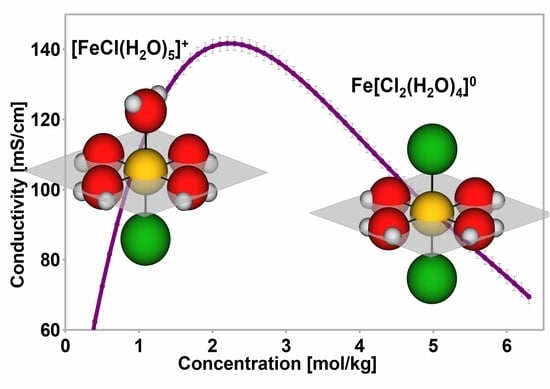
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. In Situ XAS of FeCl2 (aq) As a Function of Concentration and Temperature
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Department of Energy. Energy Storage Grand Challenge Energy Storage Market Report 2020; U.S. Department of Energy: Washington, DC, USA, 2020; Technical; p. 65.
- Comello, S.; Reichelstein, S. The emergence of cost effective battery storage. Nat. Commun. 2019, 10, 2038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bock, R.; Kleinsteinberg, B.; Selnes-Volseth, B.; Burheim, O.S. A novel iron chloride red-ox concentration flow cell battery (Icfb) concept; power and electrode optimization. Energies 2021, 14, 1109. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. Sustainable Battery Materials for Next-Generation Electrical Energy Storage. Adv. Energy Sustain. Res. 2021, 2, 2000102. [Google Scholar] [CrossRef]
- Jayathilake, B.S. Electrochemical Pathways for Sustainable Energy Storage and Energy Conversion. Ph.D. Thesis, University of Southern California, Los Angeles, CA, USA, 2019. [Google Scholar]
- Qi, Z.; Koenig, G.M. Review Article: Flow battery systems with solid electroactive materials. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2017, 35, 040801. [Google Scholar] [CrossRef] [Green Version]
- Gong, K.; Xu, F.; Grunewald, J.B.; Ma, X.; Zhao, Y.; Gu, S.; Yan, Y. All-Soluble All-Iron Aqueous Redox-Flow Battery. ACS Energy Lett. 2016, 1, 89–93. [Google Scholar] [CrossRef]
- Yensen, N.; Allen, P.B. Open source all-iron battery for renewable energy storage. HardwareX 2019, 6, e00072. [Google Scholar] [CrossRef]
- Petek, T.J.; Hoyt, N.C.; Savinell, R.F.; Wainright, J.S. Slurry electrodes for iron plating in an all-iron flow battery. J. Power Sources 2015, 294, 620–626. [Google Scholar] [CrossRef]
- Alotto, P.; Guarnieri, M.; Moro, F. Redox flow batteries for the storage of renewable energy: A review. Renew. Sustain. Energy Rev. 2014, 29, 325–335. [Google Scholar] [CrossRef]
- Valant, M. Procedure for Electric Energy Storage in Solid Matter. US Patent 10,906,805 B2, 2 February 2021. [Google Scholar]
- Kucuk, K.; Aryal, S.; Moazzen, E.; Timofeeva, E.V.; Segre, C.U. In situ XAS study of the local structure of the nano-Li2FeSiO4/C cathode. J. Phys. Energy 2021, 3, 034015. [Google Scholar] [CrossRef]
- Wang, L.; Guo, C.; Zhu, Y.; Zhou, J.; Fan, L.; Qian, Y. A FeCl2-graphite sandwich composite with Cl doping in graphite layers: A new anode material for high-performance Li-ion batteries. Nanoscale 2014, 6, 14174–14179. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, L.; Li, N.; Zhang, X. Studies of FeSe2 cathode materials for Mg-Li hybrid batteries. Energies 2020, 13, 4375. [Google Scholar] [CrossRef]
- Zhan, X.; Bowden, M.E.; Lu, X.; Bonnett, J.F.; Lemmon, T.; Reed, D.M.; Sprenkle, V.L.; Li, G. A Low-Cost Durable Na-FeCl2 Battery with Ultrahigh Rate Capability. Adv. Energy Mater. 2020, 10, 1903472. [Google Scholar] [CrossRef]
- Yang, P.; Liu, K.; Chen, Q.; Mo, X.; Zhou, Y.; Li, S.; Feng, G.; Zhou, J. Wearable Thermocells Based on Gel Electrolytes for the Utilization of Body Heat. Angew. Chem.-Int. Ed. 2016, 55, 12050–12053. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Wang, Z.; Yang, S.; Cui, X.; Li, X.; Yin, Y.; Zhang, M.; Wang, T.; Sang, S.; Zhang, W.; et al. Wearable Electronics Based on the Gel Thermogalvanic Electrolyte for Self-Powered Human Health Monitoring. ACS Appl. Mater. Interfaces 2021, 13, 37316–37322. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wu, B.; Ning, P.; Qu, G.; Li, J.; Wang, X.; Xie, R. Stabilization of arsenic in waste slag using FeCl2 or FeCl3 stabilizer. RSC Adv. 2017, 7, 54956–54963. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Brzostowski, B.; Durajski, A.; Liu, M.; Xiang, J.; Jiang, T.; Wang, Z.; Chen, S.; Li, P.; Zhong, Z.; et al. Atomically Thin 1T-FeCl 2 Grown by Molecular-Beam Epitaxy. J. Phys. Chem. C 2020, 124, 9416–9423. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, X.; Hu, L.; Gao, G. FeCl2/MoS2/FeCl2van der Waals junction for spintronic applications. J. Mater. Chem. C 2020, 8, 14353–14359. [Google Scholar] [CrossRef]
- Zhong, F.; Yang, M.; Ding, M.; Jia, C. Organic Electroactive Molecule-Based Electrolytes for Redox Flow Batteries: Status and Challenges of Molecular Design. Front. Chem. 2020, 8, 451. [Google Scholar] [CrossRef] [PubMed]
- Kamcev, J.; Sujanani, R.; Jang, E.-S.; Yan, N.; Moe, N.; Paul, D.R.; Freeman, B.D. Salt concentration dependence of ionic conductivity in ion exchange membranes. J. Memb. Sci. 2018, 547, 123–133. [Google Scholar] [CrossRef]
- Díaz, J.C.; Kamcev, J. Ionic conductivity of ion-exchange membranes: Measurement techniques and salt concentration dependence. J. Memb. Sci. 2021, 618, 118718. [Google Scholar] [CrossRef]
- Zhu, S.; Kingsbury, R.S.; Call, D.F.; Coronell, O. Impact of solution composition on the resistance of ion exchange membranes. J. Memb. Sci. 2018, 554, 39–47. [Google Scholar] [CrossRef]
- Bamgbopa, M.O.; Shao-Horn, Y.; Hashaikeh, R.; Almheiri, S. Cyclable membraneless redox flow batteries based on immiscible liquid electrolytes: Demonstration with all-iron redox chemistry. Electrochim. Acta 2018, 267, 41–50. [Google Scholar] [CrossRef]
- Geise, G.M.; Paul, D.R.; Freeman, B.D. Progress in Polymer Science Fundamental water and salt transport properties of polymeric materials. Prog. Polym. Sci. 2014, 39, 1–42. [Google Scholar] [CrossRef]
- Park, M.; Ryu, J.; Wang, W.; Cho, J. Material design and engineering of next-generation flow-battery technologies. Nat. Rev. Mater. 2016, 2, 16080. [Google Scholar] [CrossRef]
- Kuharski, R.A.; Bader, J.S.; Chandler, D.; Sprik, M.; Klein, M.L.; Impey, R.W. Molecular model for aqueous ferrous–ferric electron transfer. J. Chem. Phys. 1988, 89, 3248–3257. [Google Scholar] [CrossRef]
- Bader, J.S.; Chandler, D. Computer simulation study of the mean forces between ferrous and ferric ions in water. J. Phys. Chem. 1992, 96, 6423–6427. [Google Scholar] [CrossRef]
- Babu, C.S.; Madhusoodanan, M.; Sridhar, G.; Tembe, B.L. Orientations of [Fe(H2O)6]2+ and [Fe(H2O)6]3+ complexes at a reactive separation in water. J. Am. Chem. Soc. 1997, 119, 5679–5681. [Google Scholar] [CrossRef]
- D’Angelo, P.; Benfatto, M. Effect of Multielectronic Configurations on the XAFS Analysis at the Fe K Edge. J. Phys. Chem. A 2004, 108, 4505–4514. [Google Scholar] [CrossRef]
- Benfatto, M.; Solera, J.A.; García Ruiz, J.; Chaboy, J. Double-channel excitation in the X-ray absorption spectrum of Fe3+ water solutions. Chem. Phys. 2002, 282, 441–450. [Google Scholar] [CrossRef]
- Testemale, D.; Brugger, J.; Liu, W.; Etschmann, B.; Hazemann, J.-L. In-situ X-ray absorption study of Iron(II) speciation in brines up to supercritical conditions. Chem. Geol. 2009, 264, 295–310. [Google Scholar] [CrossRef]
- Jarzȩcki, A.A.; Anbar, A.D.; Spiro, T.G. DFT Analysis of Fe(H2O) 63+ and Fe(H2O) 62+ Structure and Vibrations; Implications for Isotope Fractionation. J. Phys. Chem. A 2004, 108, 2726–2732. [Google Scholar] [CrossRef]
- Remsungnen, T.; Rode, B.M. QM/MM Molecular Dynamics Simulation of the Structure of Hydrated Fe(II) and Fe(III) Ions. J. Phys. Chem. A 2003, 107, 2324–2328. [Google Scholar] [CrossRef]
- Ye, Q.; Chen, X.; Zhou, J.; Zhao, H.F.; Chu, W.S.; Zheng, X.S.; Marcelli, A.; Wu, Z.Y. Local hydrated structure of an Fe2+/Fe3+ aqueous solution: An investigation using a combination of molecular dynamics and X-ray absorption fine structure methods. Chin. Phys. C 2013, 37, 038003. [Google Scholar] [CrossRef]
- Lee, M.S. Chemical equilibria in ferrous chloride acid solution. Met. Mater. Int. 2004, 10, 387–392. [Google Scholar] [CrossRef]
- Ding, K.; Seyfried, W.E. Determination of Fe-Cl complexing in the low pressure supercritical region (NaCl fluid): Iron solubility constraints on pH of subseafloor hydrothermal fluids. Geochim. Cosmochim. Acta 1992, 56, 3681–3692. [Google Scholar] [CrossRef]
- Fein, J.B.; Hemley, J.J.; D’Angelo, W.M.; Komninou, A.; Sverjensky, D.A. Experimental study of iron-chloride complexing in hydrothermal fluids. Geochim. Cosmochim. Acta 1992, 56, 3179–3190. [Google Scholar] [CrossRef]
- Böhm, F.; Sharma, V.; Schwaab, G.; Havenith, M. The low frequency modes of solvated ions and ion pairs in aqueous electrolyte solutions: Iron(ii) and iron(iii) chloride. Phys. Chem. Chem. Phys. 2015, 17, 19582–19591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, R.; Pan, P. A spectrophotometric study of Fe(II)-chloride complexes in aqueous solutions from 10 to 100 °C. Can. J. Chem. 2001, 79, 131–144. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Angelo, P.; Benfatto, M.; Della Longa, S.; Pavel, N.V. Combined XANES and EXAFS analysis of Co2+, Ni2+, and Zn2+ aqueous solutions. Phys. Rev. B 2002, 66, 64209. [Google Scholar] [CrossRef]
- D’Angelo, P.; Zitolo, A.; Ceccacci, F.; Caminiti, R.; Aquilanti, G. Structural characterization of zinc(II) chloride in aqueous solution and in the protic ionic liquid ethyl ammonium nitrate by X-ray absorption spectroscopy. J. Chem. Phys. 2011, 135, 154509. [Google Scholar] [CrossRef] [PubMed]
- Dominko, R.; Sirisopanaporn, C.; Masquelier, C.; Hanzel, D.; Arčon, I.; Gaberscek, M. On the Origin of the Electrochemical Capacity of Li2Fe0.8Mn0.2{SiO}4. J. Electrochem. Soc. 2010, 157, A1309. [Google Scholar] [CrossRef]
- Roscioni, O.M.; D’Angelo, P.; Chillemi, G.; Della Longa, S.; Benfatto, M. Quantitative analysis of XANES spectra of dis{\-}ordered systems based on molecular dynamics. J. Synchrotron Radiat. 2005, 12, 75–79. [Google Scholar] [CrossRef]
- Arčon, I.; Kolar, J.; Kodre, A.; Hanžel, D.; Strlič, M. XANES analysis of Fe valence in iron gall inks. X-ray Spectrom. 2007, 36, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Chen, X.; Wang, Y.; Wu, L.; Hu, Y. Experimental and modeling of conductivity for electrolyte solution systems. ACS Omega 2020, 5, 22465–22474. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Etschmann, B.; Foran, G.J.; Shelley, M.; Brugger, J. Deriving formation constants for aqueous metal complexes from XANES spectra: Zn2+ and Fe2+ chloride complexes in hypersaline solutions. Am. Miner. 2007, 92, 761–770. [Google Scholar] [CrossRef]
- Rehr, J.J.; Albers, R.C.; Zabinsky, S.I. High-order multiple-scattering calculations of X-ray-absorption fine structure. Phys. Rev. Lett. 1992, 69, 3397–3400. [Google Scholar] [CrossRef] [PubMed]
- Bankura, A.; Santra, B.; DiStasio, R.A., Jr.; Swartz, C.W.; Klein, M.L.; Wu, X. A Systematic Study of Chloride Ion Solvation in Water using van der Waals Inclusive Hybrid Density Functional Theory. Mol. Phys. 2015, 113, 2842–2854. [Google Scholar] [CrossRef] [Green Version]
- Ge, L.; Bernasconi, L.; Hunt, P. Linking electronic and molecular structure: Insight into aqueous chloride solvation. Phys. Chem. Chem. Phys. 2013, 15, 13169–13183. [Google Scholar] [CrossRef] [Green Version]







| Fe Neighbors | N | R [Å] | σ2 [Å2] | R-Factor |
|---|---|---|---|---|
| 1 molL−1 FeCl2 aq. solution measured at RT | ||||
| O | 5.0(3) | 2.095(5) | 0.0072(5) | 0.00030 |
| Cl | 1.0 | 2.31(2) | 0.015(3) | |
| 1 molL−1 FeCl2 aq. solution measured at T = 30 °C | ||||
| O | 4.9(3) | 2.096(5) | 0.0071(5) | 0.00020 |
| Cl | 1.1 | 2.34(2) | 0.015(3) | |
| 1 molL−1 FeCl2 aq. solution measured at T = 40 °C | ||||
| O | 4.8(3) | 2.093(5) | 0.0072(5) | 0.00016 |
| Cl | 1.2 | 2.33(2) | 0.015(3) | |
| 1 molL−1 FeCl2 aq. solution measured at T = 50 °C | ||||
| O | 4.6(3) | 2.095(5) | 0.0073(5) | 0.00013 |
| Cl | 1.4 | 2.33(2) | 0.017(3) | |
| 1 molL−1 FeCl2 aq. solution measured at T = 60 °C | ||||
| O | 4.5(3) | 2.095(5) | 0.0075(5) | 0.00009 |
| Cl | 1.5 | 2.33(2) | 0.018(3) | |
| 1 molL−1 FeCl2 aq. solution measured at T = 80 °C | ||||
| O | 4.5(3) | 2.094(5) | 0.0082(5) | 0.00012 |
| Cl | 1.5 | 2.33(2) | 0.017(3) | |
| 2 molL−1 FeCl2 aq. solution measured at RT | ||||
| O | 4.5(3) | 2.103(5) | 0.0065(5) | 0.00015 |
| Cl | 1.5 | 2.35(2) | 0.021(3) | |
| 3 molL−1 FeCl2 aq. solution measured at RT | ||||
| O | 4.0(3) | 2.097(5) | 0.0066(5) | 0.00012 |
| Cl | 2.0 | 2.37(2) | 0.019(3) | |
| 4 molL−1 FeCl2 aq. solution measured at RT | ||||
| O | 4.0(3) | 2.097(5) | 0.0066(5) | 0.00012 |
| Cl | 2.0 | 2.37(2) | 0.019(3) | |
| Temperature (°C) | Average Number of Neighbors in 1 molL−1 FeCl2 | FeCl2 (aq) C. (molL−1) | Average Number of Neighbors at RT | ||
|---|---|---|---|---|---|
| Cl | O | Cl | O | ||
| 20 | 1.0 | 5.0 | 1 | 1.0 | 5.0 |
| 30 | 1.1 | 4.9 | 2 | 1.5 | 4.5 |
| 40 | 1.2 | 4.8 | 3 | 2.0 | 4.0 |
| 50 | 1.4 | 4.6 | 4 | 2.0 | 4.0 |
| 60 | 1.5 | 4.5 | |||
| 80 | 1.5 | 4.5 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luin, U.; Arčon, I.; Valant, M. Structure and Population of Complex Ionic Species in FeCl2 Aqueous Solution by X-ray Absorption Spectroscopy. Molecules 2022, 27, 642. https://doi.org/10.3390/molecules27030642
Luin U, Arčon I, Valant M. Structure and Population of Complex Ionic Species in FeCl2 Aqueous Solution by X-ray Absorption Spectroscopy. Molecules. 2022; 27(3):642. https://doi.org/10.3390/molecules27030642
Chicago/Turabian StyleLuin, Uroš, Iztok Arčon, and Matjaz Valant. 2022. "Structure and Population of Complex Ionic Species in FeCl2 Aqueous Solution by X-ray Absorption Spectroscopy" Molecules 27, no. 3: 642. https://doi.org/10.3390/molecules27030642
APA StyleLuin, U., Arčon, I., & Valant, M. (2022). Structure and Population of Complex Ionic Species in FeCl2 Aqueous Solution by X-ray Absorption Spectroscopy. Molecules, 27(3), 642. https://doi.org/10.3390/molecules27030642