ZnO Nanoparticles of Rubia cordifolia Extract Formulation Developed and Optimized with QbD Application, Considering Ex Vivo Skin Permeation, Antimicrobial and Antioxidant Properties
Abstract
:1. Introduction
2. Experimental Work
2.1. Materials
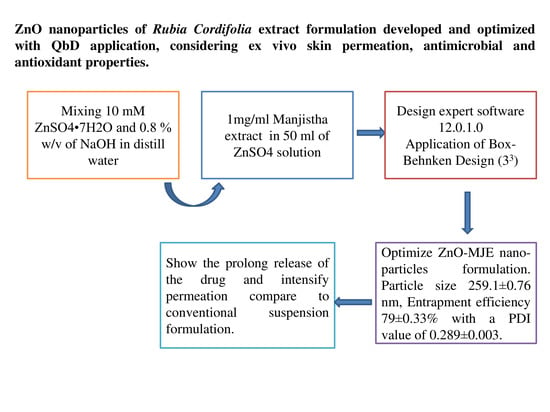
2.2. Preparation of Nanoparticles
2.3. Box–Behnken Design (BBD) Optimization
2.4. UV-Spectrophotometer Analysis
2.5. Particle Size Analysis and Morphological Characterization
2.5.1. Particle Size and Polydispersity Index (PDI)
2.5.2. Zeta Potential
2.5.3. Structural Analysis by TEM
2.5.4. Entrapment Efficiency
2.6. In Vitro Release Studies
2.7. Ex Vivo Studies
2.8. Antimicrobial Study
2.9. Antioxidant Activity
2.10. Stability Studies
2.11. Statistical Analysis
3. Results and Discussion
3.1. Optimization
3.1.1. Effect of independent variables on particle size
3.1.2. Effect of Independent Variables on Polydispersity Index (PDI)
3.1.3. Effect of Independent Variables on Entrapment Efficiency
3.1.4. Selection of Optimized Formulation
3.2. Particle Size, Zeta Potential and PDI
3.3. Morphological Examination
3.4. In Vitro Release Studies
3.5. Ex Vivo Studies
3.6. Antimicrobial Study
3.7. Antioxidant Activity
3.8. Stability Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Peterson, C.T.; Pourang, A.; Dhaliwal, S.; Kohn, J.N.; Uchitel, S.; Singh, H.; Mills, P.J.; Peterson, S.N.; Sivamani, R.K. Modulatory Effects of Triphala and Manjistha Dietary Supplementation on Human Gut Microbiota: A Double-Blind, Randomized, Placebo-Controlled Pilot Study. J. Altern. Complement. Med. 2020, 26, 1015–1024. [Google Scholar] [CrossRef]
- Shan, M.; Yu, S.; Yan, H.; Chen, P.; Zhang, L.; Ding, A. A Review of the Botany, Phytochemistry, Pharmacology and Toxicology of Rubiae Radix et Rhizoma. Molecules 2016, 21, 1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shilpa, P.N.; Venkatabalasubramanian, S.; Devaraj, S.N. Ameliorative effect of methanol extract of Rubia cordifoliain N-nitrosodiethylamine-induced hepatocellular carcinoma. Pharm. Biol. 2012, 50, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, B.S.; Prabhakara, S.; Mohan, T.; Shabeer, D.; Bhandare, B.; Nalini, M.; Sharmila, P.S.; Meghana, D.L.; Reddy, B.K.; Rao, H.H.; et al. Characterization of Rubia cordifolia L. root extract and its evaluation of cardioprotective effect in Wistar rat model. Indian J. Pharmacol. 2018, 50, 12–21. [Google Scholar] [PubMed]
- Cai, Y.; Sun, M.; Xing, J.; Corke, H. Antioxidant phenolic constituents in roots of Rheum officinale and Rubia cordifolia: Structure-radical scavenging activity relationships. J. Agric. Food Chem. 2004, 52, 7884–7890. [Google Scholar] [CrossRef]
- Shen, C.H.; Liu, C.T.; Song, X.J.; Zeng, W.Y.; Lu, X.Y.; Zheng, Z.L.; Pan, J.; Zhan, R.T.; Yan, P. Evaluation of analgesic and anti-inflammatory activities of Rubia cordifolia L. by spectrum-effect relationships. J. Chromatogr. B 2018, 1090, 73–80. [Google Scholar] [CrossRef]
- Bonifácio, B.V.; da Silva, P.B.; dos Santos Ramos, M.A.; Negri, K.M.S.; Bauab, T.M.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2014, 9, 1–15. [Google Scholar]
- Marella, S.; Tollamadugu, N.V.K.V.P. Nanotechnological approaches for the development of herbal drugs in the treatment of diabetes mellitus—A critical review. IET Nanobiotechnol. 2018, 12, 549–556. [Google Scholar] [CrossRef]
- Jahangir, M.A.; Taleuzzaman, M.; Kala, C.; Gilani, S.J. Advancements in Polymer and Lipid-based Nanotherapeutics for Cancer Drug Targeting. Curr. Pharm. Des. 2020, 26, 5119–5127. [Google Scholar] [CrossRef]
- Jahangir, M.A.; Anand, C.; Muheem, A.; Gilani, S.J.; Taleuzzaman, M.; Zafar, A.; Jafar, M.; Verma, S.; Barkat, M.A. Nano Phytomedicine Based Delivery System for CNS Disease. Curr. Drug Metab. 2020, 21, 661–673. [Google Scholar] [CrossRef]
- Namdari, M.; Eatemadi, A.; Soleimaninejad, M.; Hammed, A.T. A brief review on the application of nanoparticle enclosed herbal medicine for the treatment of infective endocarditis. Biomed. Pharmacother. 2017, 87, 321–331. [Google Scholar] [CrossRef]
- Sadeghi, F.; Ashofteh, M.; Homayouni, A.; Abbaspour, M.; Nokhodchi, A.; Garekani, H.A. Antisolvent precipitation technique: A very promising approach to crystallize curcumin in presence of polyvinyl pyrrolidon for solubility and dissolution enhancement. Colloids Surf. B Biointerfaces 2016, 147, 258–264. [Google Scholar] [CrossRef]
- Gilani, S.J.; Imam, S.S.; Ahmed, A.; Chauhan, S.; Mirza, M.A.; Taleuzzaman, M. Formulation and evaluation of thymoquinone niosomes: Application of developed and validated RP-HPLC method in the delivery system. Drug Dev. Ind. Pharm. 2019, 45, 1799–1806. [Google Scholar] [CrossRef]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Praveen, A.; Sultana, Y.; Mujeeb, M. Preparation and optimization of fisetin-loaded glycerol-based soft nanovesicles by Box-Behnken design. Int. J. Pharm. 2020, 578, 119125. [Google Scholar] [CrossRef]
- Jain, P.; Taleuzzaman, M.; Kala, C.; Gupta, D.K.; Ali, A.; Aslam, M. Quality by design (Qbd) assisted development of phytosomal gel of aloe vera extract for topical delivery. J. Liposome Res. 2021, 31, 381–388. [Google Scholar] [CrossRef]
- Sandhiya, V.; Ubaidulla, U. A review on herbal drug loaded into pharmaceutical carrier techniques and its evaluation process. Future J. Pharm. Sci. 2020, 6, 51. [Google Scholar] [CrossRef]
- Moghddam, S.M.; Ahad, A.; Aqil, M.; Imam, S.S.; Sultana, Y. Optimization of nanostructured lipid carriers for topical delivery of nimesulide using Box-Behnken design approach. Artif. Cells Nanomed. Biotechnol. 2017, 45, 617–624. [Google Scholar] [CrossRef] [Green Version]
- Nagajyothi, P.C.; Minh An, T.N.; Sreekanth, T.V.M.; Lee, J.-I.; Lee, D.J.; Lee, K.D. Green route biosynthesis: Characterization and catalytic activity of ZnO nanoparticles. Mater. Lett. 2013, 108, 160–163. [Google Scholar] [CrossRef]
- Prachi, A.M.; Mushtaq, A.; Patel, R.; Singh, N.; Negi, D.S.; Rawat, S. Green Synthesis of Zinc Oxide Nanoparticles using Rubia Cordifolia Root extract against different Bacterial Pathogens. Indo Am. J. Pharm. Res. 2017, 7, 759–765. [Google Scholar]
- Ahmad, S.; Munir, S.; Zeb, N.; Ullah, A.; Khan, B.; Ali, J.; Bilal, M.; Omer, M.; Alamzeb, M.; Salman, S.M.; et al. Green nanotechnology: A review on green synthesis of silver nanoparticles—An ecofriendly approach. Int. J. Nanomed. 2019, 14, 5087–5107. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.P.; Shukla, V.; Yadav, R.S.; Sharma, P.K.; Singh, P.; Pandey, A.C. Biological approach of zinc oxide nanoparticles formation and its characterization. Adv. Mater. Lett. 2011, 2, 313–317. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Cha, S.J.; Yang, I.J.; Sreekanth, T.V.; Kim, K.J.; Shin, H.M. Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J. Photochem. Photobiol. B Biol. 2015, 146, 10–17. [Google Scholar] [CrossRef]
- Wang, D.; Cui, L.; Chang, X.; Guan, D. Biosynthesis and characterization of zinc oxide nanoparticles from Artemisia annua and investigate their effect on proliferation, osteogenic differentiation and mineralization in human osteoblast-like MG-63 Cells. J. Photochem. Photobiol. B Biol. 2020, 202, 111652. [Google Scholar] [CrossRef]
- Mekjaruskul, C.; Sripanidkulchai, B. Kaempferia parviflora Nanosuspension Formulation for Scalability and Improvement of Dissolution Profiles and Intestinal Absorption. AAPS PharmSciTech 2020, 21, 52. [Google Scholar] [CrossRef]
- Shirsat, A.E.; Chitlange, S.S. Application of quality by design approach to optimize process and formulation parameters of rizatriptan loaded chitosan nanoparticles. J. Adv. Pharm. Technol. Res. 2015, 6, 88–96. [Google Scholar] [CrossRef]
- Bose, A.; Wong, T.W.; Singh, N. Formulation development and optimization of sustained release matrix tablet of Itopride HCl by response surface methodology and its evaluation of release kinetics. Saudi Pharm. J. 2013, 21, 201–213. [Google Scholar] [CrossRef] [Green Version]
- Monajjemzadeh, F.; Hamishehkar, H.; Zakeri-Milani, P.; Farjami, A.; Valizadeh, H. Design and optimization of sustained-release divalproex sodium tablets with response surface methodology. AAPS PharmSciTech 2013, 14, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Rosenblatt, M.K.; Bunjes, H. Evaluation of the drug loading capacity of different lipid nanoparticle dispersions by passive drug loading. Eur. J. Pharm. Biopharm. 2017, 117, 49–59. [Google Scholar] [CrossRef]
- Hao, J.; Gao, Y.; Zhao, J.; Zhang, J.; Li, Q.; Zhao, Z.; Liu, J. Preparation and optimization of resveratrol nanosuspensions by antisolvent precipitation using Box-Behnken design. AAPS PharmSciTech 2015, 16, 118–128. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Tang, S.-F.; Gao, L.-Q. Optimization of the release of salicylic acid calibrator tablets in a flow-through cell with central composite design. Chin. J. Pharm. Anal. 2009, 29, 1243–1247. [Google Scholar]
- Baláž, M.; Balážová, Ľ.; Daneu, N.; Dutková, E.; Balážová, M.; Bujňáková, Z.; Shpotyuk, Y. Plant-Mediated Synthesis of Silver Nanoparticles and Their Stabilization by Wet Stirred Media Milling. Nanoscale Res. Lett. 2017, 12, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, S.K.; Mishra, S.; Mishra, B. Eudragit-based nanosuspension of the poorly water-soluble drug: Formulation and In Vitro-In Vivo evaluation. AAPS PharmSciTech 2012, 13, 1031–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalvakuntla, S.; Deshpande, M.; Attari, Z.; Kunnatur, B.K. Preparation and Characterization of Nanosuspension of Aprepitant by H96 Process. Adv. Pharm. Bull. 2016, 6, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Caputo, F.; Clogston, J.; Calzolai, L.; Rösslein, M.; Prina-Mello, A. Measuring particle size distribution of nanoparticle enabled medicinal products, the joint view of EUNCL and NCI-NCL—A step-by-step approach combining orthogonal measurements with increasing complexity. J. Control. Release 2019, 299, 31–43. [Google Scholar] [CrossRef]
- Patel, V.R.; Agrawal, Y.K. Nanosuspension: An approach to enhance the solubility of drugs. J. Adv. Pharm. Technol. Res. 2011, 2, 81–87. [Google Scholar]
- Sathyamoorthy, N.; Magharla, D.; Chintamaneni, P.; Vankayalu, S. Optimization of paclitaxel loaded poly (ε-caprolactone) nanoparticles using Box Behnken design. Beni-Suef Univ. J. Basic Appl. Sci. 2017, 6, 362–373. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano-based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.; Ganguly, B.N. Characterization of ZnO nanoparticles grown in presence of Folic acid template. J. Nanobiotechnol. 2012, 10, 29. [Google Scholar] [CrossRef] [Green Version]
- Taleuzzaman, M.; Sartaj, A.; Gupta, D.K.; Gilani, S.J.; Mirza, M.A. Phytosomal gel of Manjistha extract (MJE) formulated and optimized with central composite design of Quality by Design (QbD). J. Dispers. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Gupta, M.; Tomar, R.S.; Kaushik, S.; Mishra, R.K.; Sharma, D. Effective Antimicrobial Activity of Green ZnO Nano Particles of Catharanthus roseus. Front. Microbiol. 2018, 9, 2030. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Sahu, A.R.; Bothara, S.B. Formulation and evaluation of phytosome drug delivery system of boswellia Serrata extract. Int. J. Res. Med. 2015, 4, 94–99. [Google Scholar]
- Moradi, S.Z.; Momtaz, S.; Bayrami, Z.; Farzaei, M.H.; Abdollahi, M. Nanoformulations of Herbal Extracts in Treatment of Neurodegenerative Disorders. Front. Bioeng. Biotechnol. 2020, 8, 238. [Google Scholar] [CrossRef]
- Parekh, J.; Chanda, S. Research Papers In Vitro antimicrobial activity of Trapa natans L. fruit rind extracted in different solvents. Afr. J. Biotechnol. 2007, 6, 766–770. [Google Scholar]
- Nayaka, H.B.; Londonkar, R.L.; Umesh, M.K. Evaluation of Potential Antifertility activity of Total Flavonoids, Isolated from Portulaca oleracea L on female albino rats. Int. J. PharmTech Res. 2014, 6, 783–793. [Google Scholar]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]









| Factor | Response | |||||
|---|---|---|---|---|---|---|
| Formulation Code (FC) | ZnSO4·7H2O (% w/v) | Ultrasonic Time (min) | Stirring Speed (rpm) | % Entrapment Efficiency ± SD (n = 3) | Particle Size ± SD (n = 3) nm | PDI ± SD (n = 3) |
| F1 | 4.75 | 2 | 500 | 81.6 ± 0.17 | 264.1 ± 0.81 | 0.291 ± 0.001 |
| F2 | 6.75 | 2 | 500 | 82.3 ± 0.05 | 263.1 ± 1.55 | 0.292 ± 0.002 |
| F3 | 4.75 | 4 | 500 | 82.85 ± 0.13 | 261.3 ± 1.62 | 0.294 ± 0.002 |
| F4 | 6.75 | 4 | 500 | 79.85 ± 0.11 | 262.3 ± 1.58 | 0.281 ± 0.003 |
| F5 | 4.75 | 3 | 400 | 78.9 ± 0.21 | 263.4 ± 0.84 | 0.279 ± 0.001 |
| F6 | 6.75 | 3 | 400 | 76.3 ± 0.29 | 257.9 ± 0.85 | 0.288 ± 0.004 |
| F7 | 4.75 | 3 | 600 | 76.1 ± 0.29 | 260.2 ± 1.13 | 0.289 ± 0.003 |
| F8 | 6.75 | 3 | 600 | 77.3 ± 0.24 | 264.5 ± 0.57 | 0.279 ± 0.001 |
| F9 | 5.75 | 2 | 400 | 78.7 ± 0.33 | 257.5 ± 0.57 | 0.319 ± 0.002 |
| F10 | 5.75 | 4 | 400 | 82.3 ± 0.24 | 260.3 ± 0.82 | 0.315 ± 0.002 |
| F11 | 5.75 | 3 | 500 | 80.08 ± 0.34 | 259.1 ± 1.16 | 0.289 ± 0.001 |
| F12 | 5.75 | 3 | 500 | 79.84 ± 0.28 | 260.1 ± 0.65 | 0.289 ± 0.002 |
| F13 * | 5.75 | 3 | 500 | 79.00 ± 0.33 | 257.1 ± 1.76 | 0.289 ± 0.003 |
| Particle Size | ||||||
|---|---|---|---|---|---|---|
| Source | Sum of Square | DF | Mean Square | F-Value | p-Value | |
| Model | 64.30 | 9 | 7.14 | 23.30 | 0.0126 | Significant |
| A-ZnSO4·7H2O | 0.1800 | 1 | 0.1800 | 0.5870 | 0.4994 | -- |
| B-Ultra-Sonication Time | 3.24 | 1 | 3.24 | 10.57 | 0.0475 | -- |
| C-Stirring Speed | 2.89 | 1 | 2.89 | 9.42 | 0.0546 | -- |
| AB | 1.00 | 1 | 1.00 | 3.26 | 0.1687 | -- |
| AC | 24.01 | 1 | 24.01 | 78.29 | 0.0030 | -- |
| BC | 7.05 | 1 | 7.05 | 23.00 | 0.0172 | -- |
| A² | 16.11 | 1 | 16.11 | 52.52 | 0.0054 | -- |
| B² | 1.52 | 1 | 1.52 | 4.95 | 0.1126 | -- |
| C² | 0.4563 | 1 | 0.4563 | 1.49 | 0.3097 | -- |
| Residual | 0.9200 | 3 | 0.3067 | -- | -- | |
| Lack of Fit | 0.1800 | 1 | 0.1800 | 0.4865 | 0.5577 | Not Significant |
| Pure Error | 0.7400 | 2 | 0.3216 | -- | -- | -- |
| Cor Total | 65.22 | 12 | -- | -- | -- | --- |
| PDI | ||||||
| Model | 0.0018 | 9 | 0.0002 | 10.50 | 0.0393 | Significant |
| A-ZnSO4·7H2O | 0.0000 | 1 | 0.0000 | 1.10 | 0.3719 | -- |
| B-Ultra-sonication time | 0.0000 | 1 | 0.0000 | 0.8306 | 0.4293 | -- |
| C-Stirring speed | 2.500 × 107 | 1 | 2.500 × 107 | 0.0130 | 0.9165 | -- |
| AB | 0.0000 | 1 | 0.0000 | 2.54 | 0.2090 | -- |
| AC | 0.0001 | 1 | 0.0001 | 4.68 | 0.1191 | -- |
| BC | 2.168 × 1019 | 1 | 2.168 × 1019 | 1.126 × 1014 | 1.0000 | -- |
| A² | 0.0006 | 1 | 0.0006 | 30.17 | 0.0119 | -- |
| B² | 0.0008 | 1 | 0.0008 | 44.08 | 0.0070 | -- |
| C² | 0.0004 | 1 | 0.0004 | 20.77 | 0.0198 | -- |
| Residual | 0.0001 | 3 | 0.0000 | -- | -- | -- |
| Lack of Fit | 0.0000 | 1 | 0.0000 | 0.7090 | 0.4884 | Not significant |
| 0.0000 | 2 | -- | -- | -- | -- | |
| 0.0019 | 12 | -- | -- | -- | -- | |
| Entrapment efficiency | ||||||
| Model | 58.83 | 9 | 6.54 | 26.34 | 0.0106 | Significant |
| A-ZnSO4·7H2O | 1.71 | 1 | 1.71 | 6.90 | 0.0786 | -- |
| B-Ultra-sonication time | 0.3600 | 1 | 0.3600 | 1.45 | 0.3148 | -- |
| C-Stirring speed | 0.8100 | 1 | 0.8100 | 3.26 | 0.1685 | -- |
| AB | 3.42 | 1 | 3.42 | 13.79 | 0.0340 | -- |
| AC | 3.61 | 1 | 3.61 | 14.55 | 0.0317 | -- |
| BC | 5.88 | 1 | 5.88 | 23.70 | 0.0166 | -- |
| A² | 0.5003 | 1 | 0.5003 | 2.02 | 0.2507 | -- |
| B² | 15.23 | 1 | 15.23 | 61.36 | 0.0043 | -- |
| C² | 10.57 | 1 | 10.57 | 42.58 | 0.0073 | -- |
| Residual | 0.7445 | 3 | 0.2482 | -- | -- | -- |
| Lack of Fit | 0.1013 | 1 | 0.1013 | 0.3148 | 0.6312 | not significant |
| Pure Error | 0.6432 | 2 | -- | -- | -- | -- |
| Cor Total | 59.58 | 12 | -- | -- | -- | -- |
| Dissolution Media | Zero Order | First Order | Higuchi Model | Korsmeyer–Peppas | ||||
|---|---|---|---|---|---|---|---|---|
| - | R2 | K | R2 | K | R2 | K | R2 | K |
| pH 7.4 | 0.8725 | 0.033 | 0.9098 | 0.000 | 0.9679 | 1.143 | 0.9912 | 4.982 |
| Months | Evaluation Parameter | |||
|---|---|---|---|---|
| Physical Appearance | Particle Size (Mean ± SD) (N = 3) | Entrapment Efficiency (Mean ± SD) (N = 3) | Drug Content (Mean ± SD) (N = 3) | |
| 0 | Clear and No Sedimentation or Cake Formation. | 262.5 ± 2.36 | 78.78 ± 2.51 | 97.26 ± 2.17 |
| 1 | Clear and No Sedimentation or Cake Formation. | 265.1 ± 4.17 | 77.89 ± 1.98 | 98.56 ± 1.88 |
| 2 | Clear and No Sedimentation or Cake Formation. | 263.7 ± 3.11 | 76.82 ± 2.28 | 97.76 ± 2.51 |
| 3 | Clear and No Sedimentation or Cake Formation. | 267.5 ± 2.78 | 76.23 ± 2.76 | 97.35 ± 2.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, J.; Anwer, M.K.; Sartaj, A.; Panda, B.P.; Ali, A.; Zafar, A.; Kumar, V.; Gilani, S.J.; Kala, C.; Taleuzzaman, M. ZnO Nanoparticles of Rubia cordifolia Extract Formulation Developed and Optimized with QbD Application, Considering Ex Vivo Skin Permeation, Antimicrobial and Antioxidant Properties. Molecules 2022, 27, 1450. https://doi.org/10.3390/molecules27041450
Kaur J, Anwer MK, Sartaj A, Panda BP, Ali A, Zafar A, Kumar V, Gilani SJ, Kala C, Taleuzzaman M. ZnO Nanoparticles of Rubia cordifolia Extract Formulation Developed and Optimized with QbD Application, Considering Ex Vivo Skin Permeation, Antimicrobial and Antioxidant Properties. Molecules. 2022; 27(4):1450. https://doi.org/10.3390/molecules27041450
Chicago/Turabian StyleKaur, Jasmeet, Md. Khalid Anwer, Ali Sartaj, Bibhu Prasad Panda, Abuzer Ali, Ameeduzzafar Zafar, Vinay Kumar, Sadaf Jamal Gilani, Chandra Kala, and Mohamad Taleuzzaman. 2022. "ZnO Nanoparticles of Rubia cordifolia Extract Formulation Developed and Optimized with QbD Application, Considering Ex Vivo Skin Permeation, Antimicrobial and Antioxidant Properties" Molecules 27, no. 4: 1450. https://doi.org/10.3390/molecules27041450
APA StyleKaur, J., Anwer, M. K., Sartaj, A., Panda, B. P., Ali, A., Zafar, A., Kumar, V., Gilani, S. J., Kala, C., & Taleuzzaman, M. (2022). ZnO Nanoparticles of Rubia cordifolia Extract Formulation Developed and Optimized with QbD Application, Considering Ex Vivo Skin Permeation, Antimicrobial and Antioxidant Properties. Molecules, 27(4), 1450. https://doi.org/10.3390/molecules27041450