Thermo-Induced Fluorochromism in Two AIE Zinc Complexes: A Deep Insight into the Structure-Property Relationship
Abstract
:1. Introduction
2. Results and Discussion
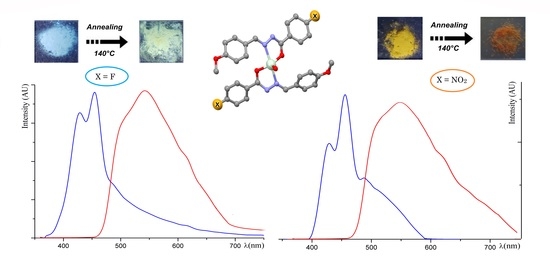
2.1. Synthesis and Spectroscopic Analysis
2.2. Crystal Structure of Ac1 and Ac2
2.3. FTIR Analysis
2.4. Theoretical Analysis
3. Experimental Section
3.1. Synthesis of the Complexes Ac1 and Ac2
3.2. Materials and Methods
3.3. PLQY Calculations
3.4. FTIR Apparatus
3.5. Single-Crystal X-ray Analysis
3.6. Molecular Modelling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Meng, K.; Yao, C.; Ma, Q.; Xue, Z.; Du, Y.; Liu, W.; Yang, D. A Reversibly Responsive Fluorochromic Hydrogel Based on Lanthanide–Mannose Complex. Adv. Sci. 2019, 6, 1802112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Zhong, H.; Chen, C.; Wu, X.-G.; Hu, X.; Huang, H.; Han, J.; Zou, B.; Dong, Y. Brightly Luminescent and Color-Tunable Colloidal CH3NH3PbX3 (X = Br, I, Cl) Quantum Dots: Potential Alternatives for Display Technology. ACS Nano 2015, 9, 4533–4542. [Google Scholar] [CrossRef] [PubMed]
- Glazer, P.J.; Leuven, J.; An, H.; Lemay, S.G.; Mendes, E. Multi-stimuli responsive hydrogel cilia. Adv. Funct. Mater. 2013, 23, 2964–2970. [Google Scholar] [CrossRef]
- Diana, R.; Panunzi, B.; Tuzi, A.; Piotto, S.; Concilio, S.; Caruso, U. An Amphiphilic Pyridinoyl-hydrazone Probe for Colorimetric and Fluorescence pH Sensing. Molecules 2019, 24, 3833. [Google Scholar] [CrossRef] [Green Version]
- Diana, R.; Panunzi, B.; Shikler, R.; Nabha, S.; Caruso, U. A symmetrical azo-based fluorophore and the derived salen multipurpose framework for emissive layers. Inorg. Chem. Commun. 2019, 104, 186–189. [Google Scholar] [CrossRef]
- Banerjee, D.; Hu, Z.; Li, J. Luminescent metal–organic frameworks as explosive sensors. Dalton Trans. 2014, 43, 10668–10685. [Google Scholar] [CrossRef]
- Bartoli, F.; Bencini, A.; Garau, A.; Giorgi, C.; Lippolis, V.; Lunghi, A.; Totti, F.; Valtancoli, B. Di- and Triphosphate Recognition and Sensing with Mono- and Dinuclear Fluorescent Zinc(II) Complexes: Clues for the Design of Selective Chemosensors for Anions in Aqueous Media. Chem. Eur. J. 2016, 22, 14890–14901. [Google Scholar] [CrossRef]
- Fan, C.; Zhang, X.; Li, N.; Xu, C.; Wu, R.; Zhu, B.; Zhang, G.; Bi, S.; Fan, Y. Zn-MOFs based luminescent sensors for selective and highly sensitive detection of Fe3+ and tetracycline antibiotic. J. Pharm. Biomed. Anal. 2020, 188, 113444. [Google Scholar] [CrossRef]
- Fan, L.; Wang, F.; Zhao, D.; Peng, Y.; Deng, Y.; Luo, Y.; Zhang, X. A self-penetrating and chemically stable zinc (ii)-organic framework as multi-responsive chemo-sensor to detect pesticide and antibiotics in water. J. Mol. Struct. 2019, 1197, 672–680. [Google Scholar] [CrossRef]
- Gabr, M.T.; Pigge, F.C. A selective fluorescent sensor for Zn2+ based on aggregation-induced emission (AIE) activity and metal chelating ability of bis(2-pyridyl)diphenylethylene. Dalton Trans. 2016, 45, 14039–14043. [Google Scholar] [CrossRef]
- Concilio, S.; Ferrentino, I.; Sessa, L.; Massa, A.; Iannelli, P.; Diana, R.; Panunzi, B.; Rella, A.; Piotto, S. A novel fluorescent solvatochromic probe for lipid bilayers. Supramol. Chem. 2017, 29, 887–895. [Google Scholar] [CrossRef]
- Prusti, B.; Samanta, P.K.; English, N.J.; Chakravarty, M. A C3-symmetric twisted organic salt as an efficient mechano-/thermo-responsive molecule: A reusable and sensitive fluorescent thermometer. Chem. Commun. 2021, 57, 12321–12324. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, M.; Chakravarty, M. Variation in solvato-, AIE- And mechano-fluorochromic behavior for furanyl and thiophenyl-substituted anthranyl π-conjugates- And role of tiny flanking donor groups. Mater. Adv. 2021, 2, 6418–6427. [Google Scholar] [CrossRef]
- Suganya, S.; Debsharma, K.; Ravindran, E.; Mahato, M.K.; Prasad, E. Phenothiazine-Based Mechano-Fluorochromic Gels and Solids: Superhydrophobic Surface Formation and Crystal-to-Crystal Phase Transition. ACS Appl. Polym. Mater. 2020, 2, 1222–1233. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Han, X.; Zhou, Y.; Lei, Y.; Gao, W.; Liu, M.; Huang, X.; Wu, H. Ketone-enol tautomerism, polymorphism, mechanofluorochromism and solid-state acidochromism of isoquinolinone-arylidenehydrazine derivatives. J. Mater. Chem. 2021, 9, 12868–12876. [Google Scholar] [CrossRef]
- Fernández-Mato, A.; Sánchez-Andújar, M.; Pato-Doldán, B.; Señarís-Rodríguez, M.A.; Platas-Iglesias, C.; Tordera, D.; Bolink, H.J.; Quintela, J.M.; Peinador, C.; García, M.D. Spontaneous Self-Assembly of a 1,8-Naphthyridine into Diverse Crystalline 1D Nanostructures: Implications on the Stimuli-Responsive Luminescent Behaviour. Cryst. Growth Des. 2014, 14, 3849–3856. [Google Scholar] [CrossRef]
- Wang, L.; Chen, H.; Yin, Q.; Kang, J.; Liu, B.; Weng, G.; He, J. Fluorochromic polymer films containing ultrasmall silver nanoclusters. Nanotechnology 2020, 31, 245703. [Google Scholar] [CrossRef]
- Guo, P.; Liu, M.; Shi, L. A Zn-based coordination polymer as a luminescent sensor for simple and sensitive detecting of sulfonamides antibiotics and nitroaromatic. J. Solid State Chem. 2020, 286, 121247. [Google Scholar] [CrossRef]
- Casalboni, M.; Caruso, U.; De Maria, A.; Fusco, M.; Panunzi, B.; Quatela, A.; Roviello, A.; Sarcinelli, F.; Sirigu, A. New polyurethanes and polyesters for second-order nonlinear optical applications. J. Polym. Sci. Part A: Polym. Chem. 2004, 42, 3013–3022. [Google Scholar]
- Klongdee, F.; Youngme, S.; Boonmak, J. A luminescent sensor based on zinc(II) 1D chain coordination polymer for effective acetone detection. Polyhedron 2020, 180, 114437. [Google Scholar] [CrossRef]
- Borbone, F.; Caruso, U.; Diana, R.; Panunzi, B.; Roviello, A.; Tingoli, M.; Tuzi, A. Second order nonlinear optical networks with excellent poling stability from a new trifunctional thiophene based chromophore. Organ. Electron. 2009, 10, 53–60. [Google Scholar] [CrossRef]
- Yu, Y.; Xing, H.; Zhou, Z.; Liu, J.; Sung, H.H.Y.; Williams, I.D.; Halpert, J.E.; Zhao, Z.; Tang, B.Z. How do molecular interactions affect fluorescence behavior of AIEgens in solution and aggregate states? Sci. China Chem. 2022, 65, 135–144. [Google Scholar] [CrossRef]
- Panunzi, B.; Concilio, S.; Diana, R.; Shikler, R.; Nabha, S.; Piotto, S.; Sessa, L.; Tuzi, A.; Caruso, U. Photophysical Properties of Luminescent Zinc(II)—Pyridinyloxadiazole Complexes and their Glassy Self-Assembly Networks. Eur. J. Inorg. Chem. 2018, 2018, 2709–2716. [Google Scholar] [CrossRef]
- Danilkina, N.A.; Andrievskaya, E.V.; Vasileva, A.V.; Lyapunova, A.G.; Rumyantsev, A.M.; Kuzmin, A.A.; Bessonova, E.A.; Balova, I.A. 4-Azidocinnoline—Cinnoline-4-amine Pair as a New Fluorogenic and Fluorochromic Environment-Sensitive Probe. Molecules 2021, 26, 7460. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, Y.; Li, Y.; Feng, Q.; Hou, H.; Tang, B.Z. 2,5-bis(4-alkoxycarbonylphenyl)-1,4-diaryl-1,4-dihydropyrrolo[3,2-b]pyrrole (AAPP) AIEgens: Tunable RIR and TICT characteristics and their multifunctional applications. Chem. Sci. 2017, 8, 7258–7267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, E.; Nagar, A.; Chaudhary, S.; Pal, S. Advanced Properties and Applications of AIEgens-Inspired Smart Materials. Ind. Eng. Chem. Res. 2020, 59, 10721–10736. [Google Scholar] [CrossRef]
- Li, B.; Zhang, D.; Li, Y.; Wang, X.; Gong, H.; Cui, Y.-Z. A reversible vapor-responsive fluorochromic molecular platform based on coupled AIE–ESIPT mechanisms and its applications in anti-counterfeiting measures. Dye. Pigment. 2020, 181, 108535. [Google Scholar] [CrossRef]
- Butler, T.; Zhuang, M.; Fraser, C.L. Color Tuning of Mechanochromic Luminescent β-Diketones via Boron Coordination and Donor-Acceptor Effects. J. Phys. Chem. 2018, 122, 19090–19099. [Google Scholar] [CrossRef]
- Hao, H.; Ye, Z.; Dai, H.; Liu, C.; Yi, A.; Xu, B.; Shi, G.; Su, S.; Azad, F.; Chi, Z. Pyrenyl-Based Aggregation-Induced Emission Luminogen for Highly Sensitive and Selective Detection of 2,4,6-Trinitrotoluene in Water. ChemistrySelect 2021, 6, 12182–12187. [Google Scholar] [CrossRef]
- Wang, R.; Diao, L.; Zhang, J.; Chen, Z.; Pu, S. Aggregation-induced emission compounds based on 9,10-dithienylanthracene and their applications in cell imaging. Dye. Pigment. 2019, 175, 108112. [Google Scholar] [CrossRef]
- Panunzi, B.; Rotiroti, L.; Tingoli, M. Solvent directed electrophilic iodination and phenylselenenylation of activated alkyl aryl ketones. Tetrahedron Lett. 2003, 44, 8753–8756. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 29, 4332–4353. [Google Scholar] [CrossRef] [PubMed]
- Kwok, R.T.K.; Leung, C.W.T.; Lam, J.W.Y.; Tang, B.Z. Biosensing by luminogens with aggregation-induced emission characteristics. Chem. Soc. Rev. 2014, 44, 4228–4238. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Ibrahim, A.-D.; Mohamed, M.A. A review on versatile applications of transition metal complexes incorporating Schiff bases. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 119–133. [Google Scholar]
- Alam, P.; Leung, N.L.; Zhang, J.; Kwok, R.T.; Lam, J.W.; Tang, B.Z. AIE-based luminescence probes for metal ion detection. Co-ord. Chem. Rev. 2020, 429, 213693. [Google Scholar] [CrossRef]
- Centore, R.; Panunzi, B.; Roviello, A.; Sirigu, A.; Villano, P. Polymers containing substituted 2-phenyl-benzoxazole side-chain groups: Synthesis and phase behavior. J. Polym. Sci. Part A: Polym. Chem. 1996, 34, 3203–3211. [Google Scholar] [CrossRef]
- Chua, M.H.; Zhou, H.; Zhu, Q.; Tang, B.Z.; Xu, J.W. Recent advances in cation sensing using aggregation-induced emission. Mater. Chem. Front. 2020, 5, 659–708. [Google Scholar] [CrossRef]
- Diana, R.; Panunzi, B. Zinc (II) and AIEgens: The “Clip Approach” for a Novel Fluorophore Family. A Review. Molecules 2021, 26, 4176. [Google Scholar] [CrossRef]
- Hirai, Y.; Laize-Générat, L.; Wrona-Piotrowicz, A.; Zakrzewski, J.; Makal, A.; Brosseau, A.; Michely, L.; Versace, D.L.; Allain, C.; Métivier, R. Multi-Directional Mechanofluorochromism of Acetyl Pyrenes and Pyrenyl Ynones. ChemPhysChem 2021, 22, 1638–1644. [Google Scholar] [CrossRef]
- Liu, Y.; Liao, Y.; Ye, Z.; Chen, L.; He, Y.; Huang, Y.; Lai, Y.; Chen, J.; Zhu, Q. Self-reversible mechanofluorochromism of AIE-active C6-unsubstituted tetrahydropyrimidine derivatives. RSC Adv. 2020, 11, 15–22. [Google Scholar] [CrossRef]
- Lei, S.N.; Xiao, H.; Zeng, Y.; Tung, C.H.; Wu, L.Z.; Cong, H. BowtieArene: A Dual Macrocycle Exhibiting Stimuli-Responsive Fluorescence. Angew. Chem. Int. Ed. 2020, 59, 10059–10065. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhu, H.; Huang, F. Alkyl Chain Length-Selective Vapor-Induced Fluorochromism of Pillar[5]arene-Based Nonporous Adaptive Crystals. J. Am. Chem. Soc. 2019, 141, 13290–13294. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, L.; Huang, J.; Zhao, J.; Yan, Y. Multifunctional Metallo-Organic Vesicles Displaying Aggregation-Induced Emission: Two-Photon Cell-Imaging, Drug Delivery, and Specific Detection of Zinc Ion. ACS Appl. Nano Mater. 2018, 1, 1819–1827. [Google Scholar] [CrossRef]
- Lescop, C. Coordination-Driven Syntheses of Compact Supramolecular Metallacycles toward Extended Metallo-organic Stacked Supramolecular Assemblies. Acc. Chem. Res. 2017, 50, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Peng, Q.; Chen, F.; Liu, Y.; Li, K.; Zang, S. AIE Ligand Constructed Zn(II) Complex with Reversible Photo-induced Color and Emission Changes. Chem. Res. Chin. Univ. 2021, 37, 123–128. [Google Scholar] [CrossRef]
- Xu, M.; Wang, X.; Wang, Q.; Hu, Q.; Huang, K.; Lou, X.; Xia, F. Analyte-responsive fluorescent probes with AIE characteristic based on the change of covalent bond. Sci. China Mater. 2019, 62, 1236–1250. [Google Scholar] [CrossRef] [Green Version]
- Caruso, U.; Diana, R.; Panunzi, B.; Roviello, A.; Tingoli, M.; Tuzi, A. Facile synthesis of new Pd(II) and Cu(II) based metallomesogens from ligands containing thiophene rings. Inorg. Chem. Commun. 2009, 12, 1135–1138. [Google Scholar] [CrossRef]
- Terenzi, A.; Lauria, A.; Almerico, A.M.; Barone, G. Zinc complexes as fluorescent chemosensors for nucleic acids: New perspectives for a “boring” element. Dalton Trans. 2014, 44, 3527–3535. [Google Scholar] [CrossRef]
- Chang, F.-F.; Zhang, K.; Huang, W. Schiff-base macrocyclic ZnII complexes based upon flexible pendant-armed extended dialdehydes. Dalton Trans. 2018, 48, 363–369. [Google Scholar] [CrossRef]
- Tian, X.; Hussain, S.; de Pace, C.; Ruiz-Pérez, L.; Battaglia, G. Zn II Complexes for Bioimaging and Correlated Applications. Chem. Asian J. 2019, 14, 509–526. [Google Scholar] [CrossRef] [Green Version]
- Jayendran, M.; Begum, P.S.; Kurup, M.P. Structural, spectral and biological investigations on Cu(II) and Zn(II) complexes derived from NNO donor tridentate Schiff base: Crystal structure of a 1D Cu(II) coordination polymer. J. Mol. Struct. 2020, 1206, 127682. [Google Scholar] [CrossRef]
- Wang, W.J.; Hao, L.; Chen, C.Y.; Qiu, Q.M.; Wang, K.; Song, J.B.; Li, H. Red-shift in fluorescence emission of D-A type asymmetrical Zn(II) complexes by extending the π-π stacking interaction. RSC Adv. 2017, 7, 20488–20493. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.-Z.; Shan, G.-G.; Li, P.; Zhou, Z.-Y.; Su, Z.-M. A novel class of Zn(II) Schiff base complexes with aggregation-induced emission enhancement (AIEE) properties: Synthesis, characterization and photophysical/electrochemical properties. Dye. Pigment. 2013, 96, 467–474. [Google Scholar] [CrossRef]
- Feng, G.; Zhang, C.-J.; Lu, X.; Liu, B. Zinc(II)-Tetradentate-Coordinated Probe with Aggregation-Induced Emission Characteristics for Selective Imaging and Photoinactivation of Bacteria. ACS Omega 2017, 2, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Kursunlu, A.N.; Ozmen, M.; Güler, E. A Novel Fluorescent Chemosensor for cu (II) Ion: Click Synthesis of Dual-Bodipy Including the Triazole Groups and Bioimaging of Yeast Cells. J. Fluoresc. 2019, 29, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Uysal, S.; Kursunlu, A.N. The Synthesis and Characterization of Star Shaped Metal Complexes of Triazine Cored Schiff Bases: Their Thermal Decompositions and Magnetic Moment Values. J. Inorg. Organomet. Polym. Mater. 2011, 21, 291–296. [Google Scholar] [CrossRef]
- Borbone, F.; Caruso, U.; Causà, M.; Fusco, S.; Panunzi, B.; Roviello, A.; Shikler, R.; Tuzi, A. Series of O,N,O-tridentate ligands zinc(II) complexes with high solid-state photoluminescence quantum yield. Eur. J. Inorg. Chem. 2014, 16, 2695–2703. [Google Scholar] [CrossRef]
- Zhang, W.; Zhong, X. Facile synthesis of ZnS-CuInS2-alloyed nanocrystals for a color-tunable fluorchrome and photocatalyst. Inorg. Chem. 2011, 50, 4065–4072. [Google Scholar] [CrossRef]
- Li, S.; Wen, H.; Yuan, N.; Xie, P.; Qin, J.; Wang, Z. Synthesis, characterization and computational studies of Zn complex based on the 8-hydroxyquinoline group containing benzimidazole. RSC Adv. 2020, 10, 32490–32496. [Google Scholar] [CrossRef]
- Miguez, F.; Menzonatto, T.G.; Netto, J.F.Z.; Souza-Silva, I.M.; Verano-Braga, T.; Lopes, J.F.; De Sousa, F.B. Photo-dynamic and fluorescent zinc complex based on spiropyran ligand. J. Mol. Struct. 2020, 1211, 128105. [Google Scholar] [CrossRef]
- Diana, R.; Panunzi, B. The Role of Zinc(II) Ion in Fluorescence Tuning of Tridentate Pincers: A Review. Molecules 2020, 25, 4984. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chi, Z.; Zhang, X.; Li, H.; Chen, C.; Liu, S.; Zhang, Y.; Xu, J. A new ligand and its complex with multi-stimuli-responsive and aggregation-induced emission effects. Chem. Commun. 2011, 47, 11080–11082. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Wang, L.; Xu, C.; Wu, R.; Li, N.; Zhang, D.; Zhang, X.; Bi, S.; Fan, Y. Synthesis, structure diversity, and dye adsorption and luminescent sensing properties of Zinc (II) coordination polymers based on 1,3,5-tris(1-imidazolyl)benzene and 1,3-bis(1-imidazolyl)toluene. J. Solid State Chem. 2020, 288, 121445. [Google Scholar] [CrossRef]
- Kursunlu, A.N. Synthesis and photophysical properties of modifiable single, dual, and triple-boron dipyrromethene (Bodipy) complexes. Tetrahedron Lett. 2015, 56, 1873–1877. [Google Scholar] [CrossRef]
- Panunzi, B.; Diana, R.; Caruso, U. A Highly Efficient White Luminescent Zinc (II) Based Metallopolymer by RGB Approach. Polymers 2019, 11, 1712. [Google Scholar] [CrossRef] [Green Version]
- Diana, R.; Panunzi, B.; Concilio, S.; Marrafino, F.; Shikler, R.; Caruso, T.; Caruso, U. The effect of bulky substituents on two π-conjugated mesogenic fluorophores. Their organic polymers and zinc-bridged luminescent networks. Polymers 2019, 11, 1379. [Google Scholar] [CrossRef] [Green Version]
- Diana, R.; Panunzi, B.; Shikler, R.; Nabha, S.; Caruso, U. Highly efficient dicyano-phenylenevinylene fluorophore as polymer dopant or zinc-driven self-assembling building block. Inorg. Chem. Commun. 2019, 104, 145–149. [Google Scholar] [CrossRef]
- Borbone, F.; Caruso, U.; Di Palma, S.; Fusco, S.; Nabha, S.; Panunzi, B.; Shikler, R. High Solid State Photoluminescence Quantum Yields and Effective Color Tuning in Polyvinylpyridine Based Zinc(II) Metallopolymers. Macromol. Chem. Phys. 2015, 216, 1516–1522. [Google Scholar] [CrossRef]
- Panunzi, B.; Borbone, F.; Capobianco, A.; Concilio, S.; Diana, R.; Peluso, A.; Piotto, S.; Tuzi, A.; Velardo, A.; Caruso, U. Synthesis, spectroscopic properties and DFT calculations of a novel multipolar azo dye and its zinc(II) complex. Inorg. Chem. Commun. 2017, 84, 103–108. [Google Scholar] [CrossRef]
- Borbone, F.; Caruso, U.; Concilio, S.; Nabha, S.; Piotto, S.; Shikler, R.; Tuzi, A.; Panunzi, B. From cadmium(II)-aroylhydrazone complexes to metallopolymers with enhanced photoluminescence. A structural and DFT study. Inorg. Chim. Acta 2017, 458, 129–137. [Google Scholar] [CrossRef]
- Diana, R.C.; Gentile, U.; Di Costanzo, F.S.; Musto, L.; Barbara Panunzi, P. Structural feature of thermo-induced fluorochromism in a 1D zinc coordination polymer. A cross-analysis by PL and FTIR spectroscopy, and DFT formalism. Dyes Pigm. 2022, 202, 110247. [Google Scholar] [CrossRef]
- Annaraj, B.; Pan, S.; Neelakantan, M.; Chattaraj, P.K. DFT study on the ground state and excited state intramolecular proton transfer of propargyl arm containing Schiff bases in solution and gas phases. Comput. Theor. Chem. 2014, 1028, 19–26. [Google Scholar] [CrossRef]
- Panunzi, B.; Diana, R.; Concilio, S.; Sessa, L.; Tuzi, A.; Piotto, S.; Caruso, U. Fluorescence pH-dependent sensing of Zn(II)by a tripodal ligand. A comparative X-ray and DFT study. J. Luminesc. 2019, 212, 200–206. [Google Scholar] [CrossRef]
- Fita, P.; Luzina, E.; Dziembowska, T.; Kopeć, D.; Piątkowski, P.; Radzewicz, C.; Grabowska, A. Keto–enol tautomerism of two structurally related Schiff bases: Direct and indirect way of creation of the excited keto tautomer. Chem. Phys. Lett. 2005, 416, 305–310. [Google Scholar] [CrossRef]
- Panunzi, B.; Diana, R.; Concilio, S.; Sessa, L.; Shikler, R.; Nabha, S.; Tuzi, A.; Caruso, U.; Piotto, S. Solid-State Highly Efficient DR Mono and Poly-dicyano-phenylenevinylene Fluorophores. Molecules 2018, 23, 1505. [Google Scholar] [CrossRef]
- Patra, L.; Das, S.; Gharami, S.; Aich, K.; Mondal, T.K. A new multi-analyte fluorogenic sensor for efficient detection of Al3+ and Zn2+ ions based on ESIPT and CHEF features. New J. Chem. 2018, 42, 19076–19082. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Argeri, M.; Borbone, F.; Caruso, U.; Causà, M.; Fusco, S.; Panunzi, B.; Roviello, A.; Shikler, R.; Tuzi, A. Color tuning and noteworthy photoluminescence quantum yields in crystalline mono-/dinuclear ZnII complexes. Eur. J. Inorg. Chem. 2014, 2014, 5916–5924. [Google Scholar] [CrossRef]
- Caruso, U.; Panunzi, B.; Diana, R.; Concilio, S.; Sessa, L.; Shikler, R.; Nabha, S.; Tuzi, A.; Piotto, S. AIE/ACQ Effects in Two DR/NIR Emitters: A Structural and DFT Comparative Analysis. Molecules 2018, 23, 1947. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yang, Z.; Song, B.; Xia, H.; Wang, Z. Syntheses, crystal structures, and fluorescent studies of Cu(II) and Zn(II) complexes bearing 2-acetonaphthonebenzoylhydrazone ligand. Inorg. Nano-Metal Chem. 2017, 47, 966–972. [Google Scholar] [CrossRef]
- Salimimarand, M.; La, D.; Al Kobaisi, M.; Bhosale, S.V. Flower-like superstructures of AIE-active tetraphenylethylene through solvophobic controlled self-assembly. Sci. Rep. 2017, 7, srep42898. [Google Scholar] [CrossRef] [PubMed]
- Salimimarand, M.; La, D.D.; Bhosale, S.V.; Jones, L.A.; Bhosale, S.V. Influence of Odd and Even Alkyl Chains on Supramolecular Nanoarchitecture via Self-Assembly of Tetraphenylethylene-Based AIEgens. Appl. Sci. 2017, 7, 1119. [Google Scholar] [CrossRef] [Green Version]
- Caruso, U.; Panunzi, B.; Roviello, G.N.; Roviello, G.; Tingoli, M.; Tuzi, A. Synthesis, structure and reactivity of amino-benzodifurane derivatives. C. R. Chim. 2009, 12, 622–634. [Google Scholar] [CrossRef]
- Zheng, H.-W.; Wu, M.; Yang, D.-D.; Liang, Q.-F.; Li, J.-B.; Zheng, X.-J. Multistimuli Responsive Solid-State Emission of a Zinc(II) Complex with Multicolour Switching. Inorg. Chem. 2021, 60, 11609–11615. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, R.; Huang, Z.; Guo, S.; Yang, J.-X.; Kong, L. A multi-stimuli-responsive tetraphenylethene derivative with high fluorescent emission in solid state. Dye. Pigment. 2021, 197, 109909. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, G.; Wang, R.; Pu, S. Highly emissive carbazole-based gold(i) complex with a long room-temperature phosphorescence lifetime and self-reversible mechanochromism characteristics. RSC Adv. 2017, 7, 15112–15115. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Yang, X.G.; Fang, X.; Wang, K.-Z.; Yan, D. Reversible Mechanochromic Delayed Fluorescence in 2D Metal-Organic Micro/Nanosheets: Switching Singlet-Triplet States through Transformation between Exciplex and Excimer. Adv. Sci. 2018, 5, 1801187. [Google Scholar] [CrossRef]
- Ren, F.; Shi, J.; Tong, B.; Cai, Z.; Dong, Y. Effects of fused rings linked to the 2,5-position of pyrrole derivatives with near-infrared emission on their aggregation-enhanced emission properties. Mater. Chem. Front. 2019, 3, 2072–2076. [Google Scholar] [CrossRef]
- Caruso, U.; Panunzi, B.; Roviello, A.; Tingoli, M.; Tuzi, A. Two aminobenzothiazole derivatives for Pd(II) and Zn(II) coordination: Synthesis, characterization and solid state fluorescence. Inorg. Chem. Commun. 2011, 14, 46–48. [Google Scholar] [CrossRef]
- Roviello, A.; Borbone, F.; Carella, A.; Diana, R.; Roviello, G.; Panunzi, B.; Ambrosio, A.; Maddalena, P. High quantum yield photoluminescence of new polyamides containing oligo-PPV amino derivatives and related oligomers. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 2677–2689. [Google Scholar] [CrossRef]
- Wei, F.; Fang, L.; Huang, Y. Synthesis, characterization, crystal structures, and photophysical properties of a series of room-temperature phosphorescent copper(I) complexes with oxadiazole-derived diimine ligand. Inorg. Chim. Acta 2010, 363, 2600–2605. [Google Scholar] [CrossRef]
- Caruso, U.; Panunzi, B.; Roviello, A.; Tuzi, A. Fluorescent metallopolymers with Zn(II) in a Schiff base/phenoxide coordination environment. Inorg. Chem. Commun. 2013, 29, 138–140. [Google Scholar] [CrossRef]
- Singh, P.; Singh, D.P.; Tiwari, K.; Mishra, M.; Singh, A.K.; Singh, V.P. Synthesis, structural investigations and corrosion inhibition studies on Mn(ii), Co(ii), Ni(ii), Cu(ii) and Zn(ii) complexes with 2-amino-benzoic acid (phenyl-pyridin-2-yl-methylene)-hydrazide. RSC Adv. 2015, 5, 45217–45230. [Google Scholar] [CrossRef]
- Hirata, S.; Head-Gordon, M. Time-dependent density functional theory within the Tamm–Dancoff approximation. Chem. Phys. Lett. 1999, 314, 291–299. [Google Scholar] [CrossRef]
- Ronca, E.; Angeli, C.; Belpassi, L.; De Angelis, F.; Tarantelli, F.; Pastore, M. Density Relaxation in Time-Dependent Density Functional Theory: Combining Relaxed Density Natural Orbitals and Multireference Perturbation Theories for an Improved Description of Excited States. J. Chem. Theory Comput. 2014, 10, 4014–4024. [Google Scholar] [CrossRef]
- Dreuw, A.A.; Head-Gordon, M. Failure of Time-Dependent Density Functional Theory for Long-Range Charge-Transfer Excited States: The Zincbacteriochlorin−Bacteriochlorin and Bacteriochlorophyll−Spheroidene Complexes. J. Am. Chem. Soc. 2004, 126, 4007–4016. [Google Scholar] [CrossRef]
- Hamel, S.; Duffy, P.; Casida, M.E.; Salahub, D.R. Kohn–Sham orbitals and orbital energies: Fictitious constructs but good approximations all the same. J. Electron Spectrosc. Relat. Phenom. 2002, 123, 345–363. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, W. A direct optimization method for calculating density functionals and exchange–correlation potentials from electron densities. J. Chem. Phys. 2003, 118, 2498–2509. [Google Scholar] [CrossRef]
- Burla, M.C.; Carrozzini, B.; Cascarano, G.L.; Giacovazzo, C.; Polidori, G. Solving proteins at non-atomic resolution by direct methods: Update. J. Appl. Crystallogr. 2017, 50, 1048–1055. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sec. Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- MacRae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, P.R. An introduction to data reduction: Space-group determination, scaling and intensity statistics. Acta Crystallogr. Sec. D Biol. Crystallogr. 2011, 67, 282–292. [Google Scholar] [CrossRef] [Green Version]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Kasha, M. Characterization of electronic transitions in complex molecules. Discuss. Faraday Soc. 1950, 9, 14–19. [Google Scholar] [CrossRef]












| λab.film (nm) [a] | λem.film (nm) [b] | Stokes Shift (nm) [c] | PLQY% [d] | CIE Coord [e] | λab.film (nm) [f] | λem.film (nm) [g] | PLQY% [h] | CIE Coord [i] | |
|---|---|---|---|---|---|---|---|---|---|
| Ac1 | 347 | 429 (455) | 92 | 21 ± 0.2 | 0.184; 0.154 | 352 | 430 (456) | 6.8 ± 0.2 | 0.25; 0.33 |
| Ac2 | 380 | 542 | 162 | 5.0 ± 0.2 | 0.421; 0.469 | 388 | 549 | 0.8 ± 0.2 | 0.57; 0.34 |
| Gap [a] (eV) | Abs [b] (nm) | Abs-M.O. [c] | Emi [b] (nm) | Emi-M.O. [c] | |
|---|---|---|---|---|---|
| Ac1 | 3.70 | 339 | H⇒L (0.64); H-1⇒L + 1 (0.33) | 439 | H⇒L (0.85); H-1⇒L + 1 (0.15) |
| Ac2 | 3.21 | 402 | H⇒L (0.94); H-1⇒L + 1 (0.06) | 504 | H⇒L (0.54); H-1⇒L + 1 (0.45) |
| Ac1 | Ac2 | |
|---|---|---|
| CCDC number | 2156626 | 2156702 |
| Formula complex and solvent | C30H24N4O4F2·Zn (II)·H2O | C30H24N6O8 Zn (II)·H2O |
| Temperature (K) | 100 | 100 |
| Wavelength (Å) | 0.7000 | 0.7000 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | Χ 2/c | Χ 2/χ |
| α (Å) | 30.629 (6) | 35.712 (11) |
| β (Å) | 8.1810 (16) | 7.690 (1) |
| χ (Å) | 10.809 (2) | 10.954 (1) |
| β (°) | 97.79 (3) | 103.703 (12) |
| R-merge (last shell: 0.75−0.71 Å) | 0.046 (0.225) | 0.038 (0.169) |
| CC (1/2) | 0.998 (0.967) | 0.999 (0.980) |
| I/σ (I) | 12.9 (3.9) | 17.4 (5.6) |
| Completeness (%) | 96.7 (95.7) | 98.5 (98.8) |
| Estimated mosaicity (°) | 0.21 | 0.25 |
| Volume | 2683.5 (9) Å3 | 2922.6 (10) Å3 |
| Z | 4 | 4 |
| Calculated density | 1.544 g/cm3 | 1.541 g/cm3 |
| θ range for data collection (°) | 2.35 to 29.754 | 1.16 to 29.742 |
| Reflections collected/unique | 19,218/3996 | 13,222/4285 |
| Data/restraints/parameters | 3996/0/202 | 4285/0/221 |
| R1 indices (I > 2σ (I), 3647) | 0.0497 (0.0646, all data) | 0.1527 (0.1660, all data) |
| wR2 | 0.153 (0.167, all data) | 0.475 (0.484, all data) |
| Highest diff. peak and hole | 1.07; −0.902 | 5.90; −0.974 |
| F (000) | 1280 | 1392 |
| Goodness-of-fit on F2 | 0.93 | 2.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diana, R.; Caruso, U.; Gentile, F.S.; Di Costanzo, L.; Musto, P.; Panunzi, B. Thermo-Induced Fluorochromism in Two AIE Zinc Complexes: A Deep Insight into the Structure-Property Relationship. Molecules 2022, 27, 2551. https://doi.org/10.3390/molecules27082551
Diana R, Caruso U, Gentile FS, Di Costanzo L, Musto P, Panunzi B. Thermo-Induced Fluorochromism in Two AIE Zinc Complexes: A Deep Insight into the Structure-Property Relationship. Molecules. 2022; 27(8):2551. https://doi.org/10.3390/molecules27082551
Chicago/Turabian StyleDiana, Rosita, Ugo Caruso, Francesco Silvio Gentile, Luigi Di Costanzo, Pellegrino Musto, and Barbara Panunzi. 2022. "Thermo-Induced Fluorochromism in Two AIE Zinc Complexes: A Deep Insight into the Structure-Property Relationship" Molecules 27, no. 8: 2551. https://doi.org/10.3390/molecules27082551
APA StyleDiana, R., Caruso, U., Gentile, F. S., Di Costanzo, L., Musto, P., & Panunzi, B. (2022). Thermo-Induced Fluorochromism in Two AIE Zinc Complexes: A Deep Insight into the Structure-Property Relationship. Molecules, 27(8), 2551. https://doi.org/10.3390/molecules27082551