Synthesis and In Vitro Characterization of Selective Cannabinoid CB2 Receptor Agonists: Biological Evaluation against Neuroblastoma Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
Synthesis of Compounds FG158a, FG160a, and FG161a
2.2. Biological Results
2.2.1. [3H]CP55,940 Binding Assays
2.2.2. CB2R Functional Activity: cAMP and β-Arrestin2 Assays
2.2.3. Investigation of Toxicity Levels against the Neuroblastoma Cells
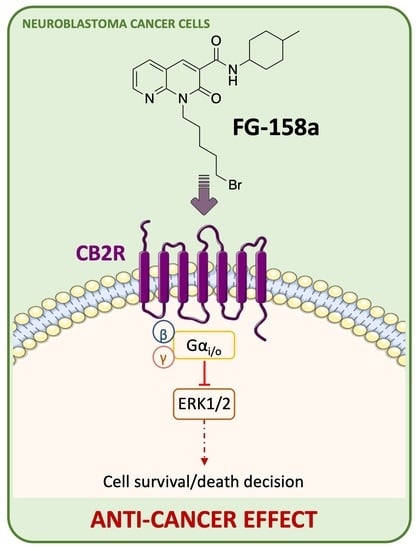
2.2.4. Effect of FG158a on ERK1/2
3. Material and Methods
3.1. Chemistry
3.1.1. Synthesis of 1-(5-bromopentyl)-N-(4-methylcyclohexyl)-2-oxo-1,2-dihydro-1,8-naphthyridine-3-carboxamide (FG158a)
3.1.2. Synthesis of 1-(5-chloropentyl)-N-(4-methylcyclohexyl)-2-oxo-1,2-dihydro-1,8-naphthyridine-3-carboxamide (FG160a)
3.1.3. Synthesis of 1-(5-azidopentyl)-N-(4-methylcyclohexyl)-2-oxo-1,2-dihydro-1,8-naphthyridine-3-carboxamide (FG161a)
3.2. Biological Assays
3.2.1. Reagents and Cell Lines
3.2.2. Radioligand Displacement Assay
3.2.3. HitHunter® cAMP Assay
3.2.4. PathHunter® CB1R β-Arrestin2 Assay
3.2.5. Sulforhodamine B (SRB) Assay
3.2.6. Electrophoresis and Immunoblots
3.2.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Maris, J.M.; Hogarty, M.D.; Bagatell, R.; Cohn, S.L. Neuroblastoma. Lancet 2007, 369, 2106–2120. [Google Scholar] [CrossRef]
- Brodeur, G.M. Spontaneous regression of neuroblastoma. Cell. Tissue Res. 2018, 372, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.K.; Dyer, M.A. Neuroblastoma: Developmental biology, cancer genomics and immunotherapy. Nat. Rev. Cancer 2013, 13, 397–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacher, P.; Kunos, G. Modulating the endocannabinoid system in human health and disease—Successes and failures. FEBS J. 2013, 280, 1918–1943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howlett, A.C.; Breivogel, C.S.; Childers, S.R.; Deadwyler, S.A.; Hampson, R.E.; Porrino, L.J. Cannabinoid Physiology and Pharmacology: 30 Years of Progress. Neuropharmacology 2004, 47, 345−358. [Google Scholar] [CrossRef]
- Turcotte, C.; Blanchet, M.R.; Laviolette, M.; Flamand, N. The CB2 receptor and Its Role as a Regulator of Inflammation. Cell. Mol. Life Sci. 2016, 73, 4449−4470. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Yang, J.; Zhao, H.; Fang, X.; Li, H. Cannabinoid receptor 2 is upregulated in melanoma. J. Cancer Res. Ther. 2012, 8, 549–554. [Google Scholar] [CrossRef]
- Bettiga, A.; Aureli, M.; Colciago, G.; Murdica, V.; Moschini, M.; Lucianò, R.; Canals, D.; Hannun, Y.; Hedlund, P.; Lavorgna, G. Bladder cancer cell growth and motility implicate cannabinoid 2 receptor-mediated modifications of sphingolipids metabolism. Sci. Rep. 2017, 7, 42157. [Google Scholar] [CrossRef] [Green Version]
- Qamri, Z.; Preet, A.; Nasser, M.W.; Bass, C.E.; Leone, G.; Barsky, S.H.; Ganju, R.K. Synthetic cannabinoid receptor agonists inhibit tumor growth and metastasis of breast cancer. Mol. Cancer Ther. 2009, 8, 3117–3129. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Martínez, E.; Gómez, I.; Martín, P.; Sánchez, A.; Román, L.; Tejerina, E.; Bonilla, F.; Merino, A.G.; Herreros, A.G.D.; Provencio, M. Cannabinoids receptor type 2, CB2, expression correlates with human colon cancer progression and predicts patient survival. Oncoscience 2015, 2, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Liu, Y.; Huang, S.; Liu, G.; Xie, C.; Zhou, J.; Fan, W.; Li, Q.; Wang, Q.; Zhong, D.; et al. Overexpression of cannabinoid receptors CB1 and CB2 correlates with improved prognosis of patients with hepatocellular carcinoma. Cancer Genet. Cytogenet. 2006, 171, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Sarfaraz, S.; Afaq, F.; Adhami, V.M.; Malik, A.; Mukhtar, H. Cannabinoid receptor agonist-induced apoptosis of human prostate cancer cells LNCaP proceeds through sustained activation of ERK1/2 leading to G1 cell cycle arrest. J. Biol. Chem. 2006, 281, 39480–39491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Y.; Huang, Y.; Zhang, Y.; Wang, C.; Wu, H.; Tian, X.; Liu, Y.; Hou, B.; Liang, Y.; Rong, H.; et al. Cannabinoid receptor 2-selective agonist JWH015 attenuates bone cancer pain through the amelioration of impaired autophagy flux induced by inflammatory mediators in the spinal cord. Mol. Med. Rep. 2019, 20, 5100–5110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almstedt, E.; Elgendy, R.; Hekmati, N.; Rosén, E.; Wärn, C.; Olsen, T.K.; Dyberg, C.; Doroszko, M.; Larsson, I.; Sundström, A.; et al. Integrative discovery of treatments for high-risk neuroblastoma. Nat. Commun. 2020, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Manera, C.; Saccomanni, G.; Malfitano, A.M.; Bertini, S.; Castelli, F.; Laezza, C.; Ligresti, A.; Lucchesi, V.; Tuccinardi, T.; Rizzolio, F.; et al. Rational design, synthesis and anti-proliferative properties of new CB2 selective cannabinoid receptor ligands: An investigation of the 1,8-naphthyridin-2(1H)-one scaffold. Eur. J. Med. Chem. 2012, 52, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, A.; Mattei, V.; Martellucci, S.; Manganelli, V.; Saccomanni, G.; Garofalo, T.; Sorice, M.; Manera, C.; Misasi, R. Anti-Proliferative Properties and Proapoptotic Function of New CB2 Selective Cannabinoid Receptor Agonist in Jurkat Leukemia Cells. Int. J. Mol. Sci. 2018, 19, 1958. [Google Scholar] [CrossRef] [Green Version]
- Manera, C.; Saccomanni, G.; Adinolfi, B.; Benetti, V.; Ligresti, A.; Cascio, M.G.; Tuccinardi, T.; Lucchesi, V.; Martinelli, A.; Nieri, P.; et al. Rational design, synthesis, and pharmacological properties of new 1,8-naphthyridin-2(1H)-on-3-carboxamide derivatives as highly selective cannabinoid-2 receptor agonists. J. Med. Chem. 2009, 52, 3644–3651. [Google Scholar] [CrossRef]
- Capozzi, A.; Caissutti, D.; Mattei, V.; Gado, F.; Martellucci, S.; Longo, A.; Recalchi, S.; Manganelli, V.; Riitano, G.; Garofalo, T.; et al. Anti-Inflammatory Activity of a CB2 Selective Cannabinoid Receptor Agonist: Signaling and Cytokines Release in Blood Mononuclear Cells. Molecules 2022, 27, 64. [Google Scholar] [CrossRef]
- Malfitano, A.M.; Laezza, C.; D’Alessandro, A.; Procaccini, C.; Saccomanni, G.; Tuccinardi, T.; Manera, C.; Macchia, M.; Matarese, G.; Gazzerro, P.; et al. Effects on immune cells of a new 1,8-naphthyridin-2-one derivative and its analogues as selective CB2 agonists: Implications in multiple sclerosis. PLoS ONE 2013, 8, e62511. [Google Scholar] [CrossRef] [Green Version]
- Malfitano, A.M.; Proto, M.C.; Bifulco, M. Cannabinoids in the management of spasticity associated with multiple sclerosis. Neuropsychiatr. Dis. Treat. 2008, 4, 847–853. [Google Scholar]
- Madaan, A.; Verma, R.; Kumar, V.; Singh, A.T.; Jain, S.K.; Jaggi, M. 1,8-Naphthyridine Derivatives: A Review of Multiple Biological Activities. Arch. Pharm. 2015, 348, 837–860. [Google Scholar] [CrossRef]
- Shwetha, B.; Sudhanva, M.S.; Jagadeesha, G.S.; Thimmegowda, N.R.; Hamse, V.K.; Sridhar, B.T.; Thimmaiah, K.N.; Ananda Kumar, C.S.; Shobith, R.; Rangappa, K.S. Furan-2-carboxamide derivative, a novel microtubule stabilizing agent induces mitotic arrest and potentiates apoptosis in cancer cells. Bioorg. Chem. 2021, 108, 104586. [Google Scholar] [CrossRef] [PubMed]
- Hawash, M.; Kahraman, D.C.; Olgac, A.; Ergun, S.G.; Hamel, E.; Cetin-Atalay, R.; Baytas, S.N. Design and synthesis of novel substituted indole-acrylamide derivatives and evaluation of their anti-cancer activity as potential tubulin-targeting agents. J. Mol. Struct. 2022, 1254, 132345. [Google Scholar] [CrossRef]
- Hawash, M.; Jaradat, N.; Bawwab, N.; Salem, K.; Arafat, H.; Hajyousef, Y.; Shtayeh, T.; Sobuh, S. Design, synthesis, and biological evaluation of phenyl-isoxazole-carboxamide derivatives as anticancer agents. Heterocycl. Comm. 2021, 27, 133–141. [Google Scholar] [CrossRef]
- Xu, X.; Rajamanickam, V.; Shu, S.; Liu, Z.; Yan, T.; He, J.; Liu, Z.; Guo, G.; Liang, G.; Wang, Y. Indole-2-Carboxamide Derivative LG25 Inhibits Triple-Negative Breast Cancer Growth By Suppressing Akt/mTOR/NF-κB Signalling Pathway. Drug Des. Dev. Ther. 2019, 13, 3539–3550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucchesi, V.; Hurst, D.P.; Shore, D.M.; Bertini, S.; Ehrmann, B.M.; Allarà, M.; Lawrence, L.; Ligresti, A.; Minutolo, F.; Saccomanni, G.; et al. CB2-selective cannabinoid receptor ligands: Synthesis, pharmacological evaluation, and molecular modeling investigation of 1,8-Naphthyridin-2(1H)-one-3-carboxamides. J. Med. Chem. 2014, 57, 8777–8791. [Google Scholar] [CrossRef] [Green Version]
- Wojcieszak, J.; Krzemień, W.; Zawilska, J.B. JWH-133, a Selective Cannabinoid CB₂ Receptor Agonist, Exerts Toxic Effects on Neuroblastoma SH-SY5Y Cells. J. Mol. Neurosci. 2016, 58, 441–445. [Google Scholar] [CrossRef]
- Hanlon, K.E.; Lozano-Ondoua, A.N.; Umaretiya, P.J.; Symons-Liguori, A.M.; Chandramouli, A.; Moy, J.K.; Kwass, W.K.; Mantyh, P.W.; Nelson, M.A.; Vanderah, T.W. Modulation of breast cancer cell viability by a cannabinoid receptor 2 agonist, JWH-015, is calcium dependent. Breast Cancer 2016, 8, 59–71. [Google Scholar]
- Eleveld, T.F.; Oldridge, D.A.; Bernard, V.; Koster, J.; Colmet Daage, L.; Diskin, S.J.; Schild, L.; Bentahar, N.B.; Bellini, A.; Chicard, M.; et al. Relapsed neuroblastomas show frequent RAS-MAPK pathway mutations. Nat. Genet. 2015, 47, 864–871. [Google Scholar] [CrossRef] [Green Version]
- Zagzoog, A.; Mohamed, K.A.; Kim, H.J.J.; Kim, E.D.; Frank, C.S.; Black, T.; Jadhav, P.D.; Holbrook, L.A.; Laprairie, R.B. In vitro and in vivo pharmacological activity of minor cannabinoids isolated from Cannabis sativa. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Di Natale, C.; La Manna, S.; Malfitano, A.M.; Di Somma, S.; Florio, D.; Scognamiglio, P.L.; Novellino, E.; Netti, P.A.; Marasco, D. Structural insights into amyloid structures of the C-terminal region of nucleophosmin 1 in type A mutation of acute myeloid leukemia. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Di Somma, S.; Iannuzzi, C.A.; Passaro, C.; Forte, I.M.; Iannone, R.; Gigantino, V.; Indovina, P.; Botti, G.; Giordano, A.; Formisano, P.; et al. The Oncolytic Virus dl 922-947 Triggers Immunogenic Cell Death in Mesothelioma and Reduces Xenograft Growth. Front. Oncol. 2019, 9, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawash, M.M.; Kahraman, D.C.; Eren, F.; Cetin Atalay, R.; Baytas, S.N. Synthesis and biological evaluation of novel pyrazolic chalcone derivatives as novel hepatocellular carcinoma therapeutics. Eur. J. Med. Chem. 2017, 129, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Laezza, C.; D’Alessandro, A.; Di Croce, L.; Picardi, P.; Ciaglia, E.; Pisanti, S.; Malfitano, A.M.; Comegna, M.; Faraonio, R.; Gazzerro, P.; et al. p53 regulates the mevalonate pathway in human glioblastoma multiforme. Cell. Death Dis. 2015, 6, e1909. [Google Scholar] [CrossRef] [Green Version]
- Bifulco, M.; D’Alessandro, A.; Paladino, S.; Malfitano, A.M.; Notarnicola, M.; Caruso, M.G.; Laezza, C. N6-isopentenyladenosine improves nuclear shape in fibroblasts from humans with progeroid syndromes by inhibiting the farnesylation of prelamin A. FEBS J. 2013, 280, 6223–6232. [Google Scholar] [CrossRef]







| Cmpds | CB2R Ki (nM) 1 | CB1R Ki (nM) 1 | cAMP Inhibition | β-Arrestin2 Recruitment | ||
|---|---|---|---|---|---|---|
| EC50 (95% C.I., nM) | Emax (% CP55,940) ± S.E.M | EC50 (95% C.I., nM) | Emax (% CP55,940) ± S.E.M | |||
| CP55,940 | 34 (2.7–57) | 6.6 (2.7–15) | 9.4 (3.4–29)) | 100 ± 6.4 | 560 (410–760) | 100 ± 3.4 |
| FG158a | 21 (12–50) | >10,000 | >10,000 | 71 ± 2.5 * | >10,000 | 46 ± 1.8 * |
| FG160a | 16.5 (10–45) | >10,000 | 600 (73–870) * | 90 ± 3.1 | >10,000 | 41 ± 1.0 * |
| FG161a | 45 (32–75) | >10,000 | 760 (150–2,900) * | 78 ± 11 | >10,000 | 47 ± 1.8 * |
| LV62 | 37 (13–89) | >10,000 | 34 (3.9–220) | 110 ± 8.8 | 63 (49–82) * | 65 ± 1.4 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gado, F.; Ferrisi, R.; Di Somma, S.; Napolitano, F.; Mohamed, K.A.; Stevenson, L.A.; Rapposelli, S.; Saccomanni, G.; Portella, G.; Pertwee, R.G.; et al. Synthesis and In Vitro Characterization of Selective Cannabinoid CB2 Receptor Agonists: Biological Evaluation against Neuroblastoma Cancer Cells. Molecules 2022, 27, 3019. https://doi.org/10.3390/molecules27093019
Gado F, Ferrisi R, Di Somma S, Napolitano F, Mohamed KA, Stevenson LA, Rapposelli S, Saccomanni G, Portella G, Pertwee RG, et al. Synthesis and In Vitro Characterization of Selective Cannabinoid CB2 Receptor Agonists: Biological Evaluation against Neuroblastoma Cancer Cells. Molecules. 2022; 27(9):3019. https://doi.org/10.3390/molecules27093019
Chicago/Turabian StyleGado, Francesca, Rebecca Ferrisi, Sarah Di Somma, Fabiana Napolitano, Kawthar A. Mohamed, Lesley A. Stevenson, Simona Rapposelli, Giuseppe Saccomanni, Giuseppe Portella, Roger G. Pertwee, and et al. 2022. "Synthesis and In Vitro Characterization of Selective Cannabinoid CB2 Receptor Agonists: Biological Evaluation against Neuroblastoma Cancer Cells" Molecules 27, no. 9: 3019. https://doi.org/10.3390/molecules27093019
APA StyleGado, F., Ferrisi, R., Di Somma, S., Napolitano, F., Mohamed, K. A., Stevenson, L. A., Rapposelli, S., Saccomanni, G., Portella, G., Pertwee, R. G., Laprairie, R. B., Malfitano, A. M., & Manera, C. (2022). Synthesis and In Vitro Characterization of Selective Cannabinoid CB2 Receptor Agonists: Biological Evaluation against Neuroblastoma Cancer Cells. Molecules, 27(9), 3019. https://doi.org/10.3390/molecules27093019