Ameliorative Effect of Structurally Divergent Oleanane Triterpenoid, 3-Epifriedelinol from Ipomoea batatas against BPA-Induced Gonadotoxicity by Targeting PARP and NF-κB Signaling in Rats
Abstract
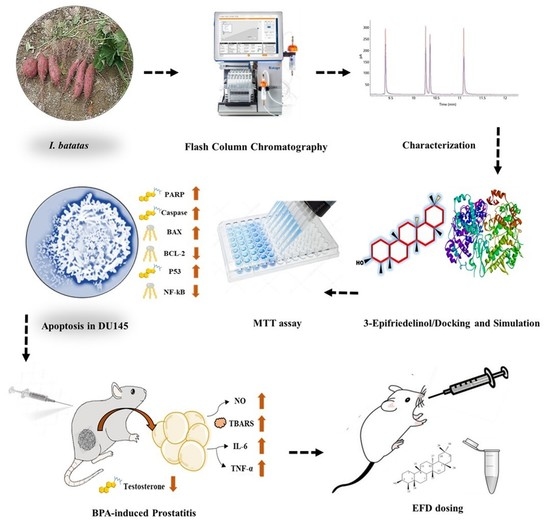
:1. Introduction
2. Results
2.1. Structure Elucidation of EFD
2.2. Molecular Docking
2.3. Antiproliferative Potential of EFD
2.4. EFD Induces Apoptosis
2.5. Size, Weight and ROW of Gonads
2.6. Effect on Hematology and Histology
2.7. Effect of EFD on Gonadal Hormones
2.8. Effect of EFD on Endogenous Enzymes and Inflammatory Markers
2.9. Pharmacokinetic and ADMET Properties
3. Material and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Samples
3.3. Molecular Docking
3.4. Cytotoxicity against Prostate Cancer Cell Line
3.5. Western Blotting
3.6. Animals
3.7. Experimental Design
3.8. The Gonads’ Size, Weight, and Relative Organ Weight (ROW)
3.9. Hematological Parameters
3.10. Hormonal and Biochemical Assessment
3.11. Endogenous Enzymes and Pro-Inflammatory Markers
3.12. ADMET Predictions
3.13. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Muhammad, I.; Xiao, Y.Z.; Hassan, S.S.U.; Xiao, X.; Yan, S.K.; Guo, Y.Q.; Ma, X.P.; Jin, H.Z. Three New Guaiane-Type Sesquiterpenoids and a Monoterpenoid from Litsea Lancilimba Merr. Nat. Prod. Res. 2020, 36, 3271–3279. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, I.; Hassan, S.S.U.; Cheung, S.; Li, X.; Wang, R.; Zhang, W.D.; Yan, S.K.; Zhang, Y.; Jin, H.Z. Phytochemical Study of Ligularia Subspicata and Valuation of Its Anti-Inflammatory Activity. Fitoterapia 2021, 148, 104800. [Google Scholar] [CrossRef] [PubMed]
- Memariani, Z.; Abbas, S.Q.; Hassan, S.S.U.; Ahmadi, A.; Chabra, A. Naringin and Naringenin as Anticancer Agents and Adjuvants in Cancer Combination Therapy: Efficacy and Molecular Mechanisms of Action, a Comprehensive Narrative Review. Pharmacol. Res. 2021, 171, 105264. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.; Hamid, K.; Kam, A.; Wong, K.H.; Abdelhak, Z.; Razmovski-Naumovski, V.; Chan, K.; Li, K.M.; Groundwater, P.W.; Li, G.Q. The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications. Curr. Med. Chem. 2013, 20, 908–931. [Google Scholar] [PubMed]
- Dargahi, N.; Johnson, J.C.; Apostolopoulos, V. Immune Modulatory Effects of Probiotic Streptococcus thermophilus on Human Monocytes. Biologics 2021, 1, 396–415. [Google Scholar] [CrossRef]
- Ağagündüz, D.; Kocaadam-Bozkurt, B.; Bozkurt, O.; Sharma, H.; Esposito, R.; Özoğul, F.; Capasso, R. Microbiota alteration and modulation in Alzheimer’s disease by gerobiotics: The gut-health axis for a good mind. Biomed. Pharmacother. 2022, 153, 113430. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Crawford, E.D. Understanding the epidemiology, natural history, and key pathways involved in prostate cancer. Urology 2009, 73, S4–S10. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.M.; Al-Kahtani, M.A.; El-Kersh, M.A.; Al-Omair, M.A. Free radical-scavenging, anti-inflammatory/anti-fibrotic and hepatoprotective actions of taurine and silymarin against CCl4 induced rat liver damage. PLoS ONE 2015, 10, e0144509. [Google Scholar] [CrossRef] [Green Version]
- Mínguez-Alarcón, L.; Hauser, R.; Gaskins, A.J. Effects of bisphenol A on male and couple reproductive health: A review. Fertil. Steril. 2016, 106, 864–870. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, Y.-Y.; Liu, L.; Qiao, Y.-N.; Geng, H.-R.; Lin, Y.; Xu, W.; Cao, J.; Zhao, J.-Y. Homocysteine Inhibits Pro-Insulin Receptor Cleavage and Causes Insulin Resistance via Protein Cysteine-Homocysteinylation. Cell Rep. 2021, 37, 109821. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.W.; Nasir, B.; Waseem, D.; Majid, M.; Khan, M.Z.I.; Haq, I.-U. Withametelin: A biologically active withanolide in cancer, inflammation, pain and depression. Saudi Pharm. J. 2020, 28, 1526–1537. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-H.; Xu, S.; Zhou, X.-Y.; Zhao, R.; Lin, Y.; Cao, J.; Zang, W.-D.; Tao, H.; Xu, W.; Li, M.-Q.; et al. Low Chorionic Villous Succinate Accumulation Associates with Recurrent Spontaneous Abortion Risk. Nat. Commun. 2021, 12, 3428. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.-Y.; Zhao, R.; Zhang, H.-L.; Zhou, Q.; Xu, F.-J.; Zhang, X.; Xu, W.-H.; Shao, N.; Zhou, S.-X.; Dai, B.; et al. Inactivation of the AMPK-GATA3-ECHS1 Pathway Induces Fatty Acid Synthesis That Promotes Clear Cell Renal Cell Carcinoma Growth. Cancer Res. 2020, 80, 319–333. [Google Scholar] [CrossRef] [Green Version]
- Majid, M.; Nasir, B.; Zahra, S.S.; Khan, M.R.; Mirza, B.; Haq, I.-U. Ipomoea batatas L. Lam. ameliorates acute and chronic inflammations by suppressing inflammatory mediators, a comprehensive exploration using in vitro and in vivo models. BMC Complement. Altern. Med. 2018, 18, 216. [Google Scholar] [CrossRef]
- Li, Y.; Liao, Z.; Wei, X.; Xiao, X.; Hu, J. Epifriedelanol enhances adriamycin-induced cytotoxicity towards K562/ADM cells by down regulating of P-gp and MRP2. Xenobiotica 2022, 52, 389–396. [Google Scholar] [CrossRef]
- Perumal, P.C.; Sowmya, S.; Velmurugan, D.; Sivaraman, T.; Gopalakrishnan, V.K. Assessment of dual inhibitory activity of epifriedelanol isolated from Cayratia trifolia against ovarian cancer. Bangladesh J. Pharmacol. 2016, 11, 545–551. [Google Scholar] [CrossRef]
- Kundu, J.; Rouf, A.; Hossain, M.N.; Hasan, C.; Rashid, M. Antitumor activity of epifriedelanol from Vitis trifolia. Fitoterapia 2000, 71, 577–579. [Google Scholar] [CrossRef]
- Yang, H.H.; Son, J.-K.; Jung, B.; Zheng, M.; Kim, J.-R. Epifriedelanol from the root bark of Ulmus davidiana inhibits cellular senescence in human primary cells. Planta Med. 2011, 77, 441–449. [Google Scholar] [CrossRef] [Green Version]
- Shams, S.; Zhang, W.; Jin, H.; Basha, S.H.; Priya, S.V.S.S. In-Silico Anti-Inflammatory Potential of Guaiane Dimers from Xylopia Vielana Targeting COX-2. J. Biomol. Struct. Dyn. 2020, 40, 484–498. [Google Scholar] [CrossRef]
- Hassan, S.S.U.; Abbas, S.Q.; Hassan, M.; Jin, H.-Z. Computational Exploration of Anti-Cancer Potential of Guaiane Dimers from Xylopia Vielana by Targeting B-Raf Kinase Using Chemo-Informatics, Molecular Docking and MD Simulation Studies. Anti-Cancer Agents Med. Chem. 2022, 22, 731–746. [Google Scholar] [CrossRef]
- Majid, M.; Farhan, A.; Asad, M.I.; Khan, M.R.; Hassan, S.S.U.; Haq, I.-U.; Bungau, S. An Extensive Pharmacological Evaluation of New Anti-Cancer Triterpenoid (Nummularic Acid) from Ipomoea batatas through In Vitro, In Silico, and In Vivo Studies. Molecules 2022, 27, 2474. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Tao, H.; Cao, W.; Cao, L.; Lin, Y.; Zhao, S.-M.; Xu, W.; Cao, J.; Zhao, J.-Y. Ketogenic Diets Inhibit Mitochondrial Biogenesis and Induce Cardiac Fibrosis. Signal Transduct. Target. Ther. 2021, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Majid, M.; Ijaz, F.; Baig, M.W.; Nasir, B.; Khan, M.R.; Haq, I.-U. Scientific Validation of Ethnomedicinal Use of Ipomoea batatas L. Lam. as Aphrodisiac and Gonadoprotective Agent against Bisphenol A Induced Testicular Toxicity in Male Sprague Dawley Rats. BioMed Res. Int. 2019, 2019, 8939854. [Google Scholar] [CrossRef] [PubMed]
- Ola-Mudathir, K.F.; Suru, S.M.; Fafunso, M.A.; Obioha, U.E.; Faremi, T.Y. Protective roles of onion and garlic extracts on cadmium-induced changes in sperm characteristics and testicular oxidative damage in rats. Food Chem. Toxicol. 2008, 46, 3604–3611. [Google Scholar] [CrossRef] [PubMed]
- Majid, M.; Khan, M.R.; Shah, N.A.; Haq, I.U.; Farooq, M.A.; Ullah, S.; Sharif, A.; Zahra, Z.; Younis, T.; Sajid, M. Studies on phytochemical, antioxidant, anti-inflammatory and analgesic activities of Euphorbia dracunculoides. BMC Complement. Altern. Med. 2015, 15, 349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasir, B.; Baig, M.W.; Majid, M.; Ali, S.M.; Khan, M.Z.I.; Kazmi, S.T.B.; Haq, I.-U. Preclinical anticancer studies on the ethyl acetate leaf extracts of Datura stramonium and Datura inoxia. BMC Complement. Med. Ther. 2020, 20, 188. [Google Scholar] [CrossRef]
- Hassan, S.S.U.; Abbas, S.Q.; Muhammad, I.; Wu, J.J.; Yan, S.K.; Ali, F.; Majid, M.; Jin, H.Z.; Bungau, S. Metals-triggered compound CDPDP exhibits anti-arthritic behavior by downregulating the inflammatory cytokines, and modulating the oxidative storm in mice models with extensive ADMET, docking and simulation studies. Front. Pharmacol. 2022, 13, 1053744. [Google Scholar] [CrossRef]
- Ren, Y.; Anaya-Eugenio, G.D.; Czarnecki, A.A.; Ninh, T.N.; Yuan, C.; Chai, H.-B.; Soejarto, D.D.; Burdette, J.E.; de Blanco, E.J.C.; Kinghorn, A.D. Cytotoxic and NF-κB and mitochondrial transmembrane potential inhibitory pentacyclic triterpenoids from Syzygium corticosum and their semi-synthetic derivatives. Bioorganic Med. Chem. 2018, 26, 4452–4460. [Google Scholar] [CrossRef]
- Li, Y.; Yao, C.-F.; Xu, F.-J.; Qu, Y.-Y.; Li, J.-T.; Lin, Y.; Cao, Z.-L.; Lin, P.-C.; Xu, W.; Zhao, S.-M.; et al. APC/C(CDH1) Synchronizes Ribose-5-Phosphate Levels and DNA Synthesis to Cell Cycle Progression. Nat. Commun. 2019, 10, 2502. [Google Scholar] [CrossRef]
- Moghtaderi, H.; Sepehri, H.; Attari, F. Combination of arabinogalactan and curcumin induces apoptosis in breast cancer cells in vitro and inhibits tumor growth via overexpression of p53 level in vivo. Biomed. Pharmacother. 2017, 88, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.A.R.; Leal, A.S.; Valdeira, A.S.; Gonçalves, B.M.F.; Alho, D.P.S.; Figueiredo, S.A.C.; Silvestre, S.M.; Mendes, V.I.S. Oleanane-, ursane-, and quinone methide friedelane-type triterpenoid derivatives: Recent advances in cancer treatment. Eur. J. Med. Chem. 2017, 142, 95–130. [Google Scholar] [CrossRef] [PubMed]
- Maiti, D.; Singha, A.K.; Sarkar, C.; Sarkar, S.; Sil Sarma, I.; Manna, K.; Dinda, B. Friedelane, isolated from Pouzolzia indica Gaud. exhibits toxic effect against melanoma. Cytotechnology 2018, 70, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Yang, Z.; Fan, Y.; Gong, L.; Han, Z.; Ji, L.; Hu, X.; Wu, D. Gut Microbiota on Admission as Predictive Biomarker for Acute Necrotizing Pancreatitis. Front. Immunol. 2022, 13, 988326. [Google Scholar] [CrossRef]
- Ferrarini, E.G.; Paes, R.S.; Baldasso, G.M.; de Assis, P.M.; Gouvêa, M.C.; De Cicco, P.; Raposo, N.R.B.; Capasso, R.; Moreira, E.L.G.; Dutra, R.C. Broad-spectrum cannabis oil ameliorates reserpine-induced fibromyalgia model in mice. Biomed. Pharmacother. 2022, 154, 113552. [Google Scholar] [CrossRef]
- Yang, J.; Fa, J.; Li, B. Apoptosis induction of epifriedelinol on human cervical cancer cell line. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Ng, T.B.; Liu, F.; Lu, Y.; Cheng, C.; Wang, Z. Antioxidant activity of compounds from the medicinal herb Aster tataricus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 136, 109–115. [Google Scholar] [CrossRef]
- Zhang, H.-T.; Tian, M.; He, Q.-W.; Chi, N.; Xiu, C.-M.; Wang, Y.-B. Effect of Aster tataricus on production of inflammatory mediators in LPS stimulated rat astrocytoma cell line (C6) and THP-1 cells. Saudi Pharm. J. 2017, 25, 370–375. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Q.; Bie, B.; Guo, Q.; Yuan, Y.; Han, Q.; Han, X.; Chen, M.; Zhang, X.; Yang, Y.; Liu, M.; et al. Hyperpolarized Xe NMR Signal Advancement by Metal-Organic Framework Entrapment in Aqueous Solution. Proc. Natl. Acad. Sci. USA 2020, 117, 17558–17563. [Google Scholar] [CrossRef]
- Rengarajan, T.; Nandakumar, N.; Rajendran, P.; Haribabu, L.; Nishigaki, I.; Balasubramanian, M.P. D-pinitol promotes apoptosis in MCF-7 cells via induction of p53 and Bax and inhibition of Bcl-2 and NF-κB. Asian Pac. J. Cancer Prev. 2014, 15, 1757–1762. [Google Scholar] [CrossRef]
- Yeh, C.T.; Wu, C.H.; Yen, G.C. Ursolic acid, a naturally occurring triterpenoid, suppresses migration and invasion of human breast cancer cells by modulating c-Jun N-terminal kinase, Akt and mammalian target of rapamycin signaling. Mol. Nutr. Food Res. 2010, 54, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.-Z.; Xuan, Y.-Y.; Ruan, S.-Q.; Sun, M. Proliferation-inhibiting and apoptosis-inducing effects of ursolic acid and oleanolic acid on multi-drug resistance cancer cells in vitro. Chin. J. Integr. Med. 2011, 17, 607. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Li, T.; Tian, J.X.; Xi, P.; Liu, R.H. Ursolic acid, a potential anticancer compound for breast cancer therapy. Crit. Rev. Food Sci. Nutr. 2018, 58, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Cheng, J.; Liu, Y.; Cheng, C.; Zhang, M.; Sui, W.; Chen, W.; Hao, P.; Zhang, Y.; Zhang, C. Cardiomyocyte-Specific Knockout of ADAM17 Ameliorates Left Ventricular Remodeling and Function in Diabetic Cardiomyopathy of Mice. Signal Transduct. Target. Ther. 2022, 7, 259. [Google Scholar] [CrossRef] [PubMed]
- Topçu, G. Bioactive triterpenoids from Salvia species. J. Nat. Prod. 2006, 69, 482–487. [Google Scholar] [CrossRef]
- Sato, H.; Macchiarulo, A.; Thomas, C.; Gioiello, A.; Une, M.; Hofmann, A.F.; Saladin, R.; Schoonjans, K.; Pellicciari, R.; Auwerx, J. Novel Potent and Selective Bile Acid Derivatives as TGR5 Agonists: Biological Screening, Structure−Activity Relationships, and Molecular Modeling Studies. J. Med. Chem. 2008, 51, 1831–1841. [Google Scholar] [CrossRef]
- Ayyanar, M.; Subash-Babu, P. Syzygium cumini (L.) Skeels: A review of its phytochemical constituents and traditional uses. Asian Pac. J. Trop. Biomed. 2012, 2, 240–246. [Google Scholar] [CrossRef] [Green Version]
- Olasantan, O.; Areola, J.; Ayannuga, O.; Babalola, O. Evaluation of the gonadoprotective effects of Allanblackia floribundaOliver (Clusiacea) on testes and accessory organs of Wistar rats. J. Med. Biol. Sci. Res. 2015, 1, 134–144. [Google Scholar]
- Baig, M.W.; Majid, M.; Nasir, B.; Hassan, S.S.U.; Bungau, S.; Haq, I.-U. Toxicity evaluation induced by single and 28-days repeated exposure of withametelin and daturaolone in Sprague Dawley rats. Front. Pharmacol. 2022, 13, 999078. [Google Scholar] [CrossRef]
- Moon, M.K.; Kim, M.J.; Jung, I.K.; Koo, Y.D.; Ann, H.Y.; Lee, K.J.; Kim, S.H.; Yoon, Y.C.; Cho, B.-J.; Park, K.S. Bisphenol A impairs mitochondrial function in the liver at doses below the no observed adverse effect level. J. Korean Med. Sci. 2012, 27, 644–652. [Google Scholar] [CrossRef] [Green Version]
- Zahra, Z.; Khan, M.R.; Majid, M.; Maryam, S.; Sajid, M. Gonadoprotective ability of Vincetoxicum arnottianum extract against bisphenol A-induced testicular toxicity and hormonal imbalance in male Sprague Dawley rats. Andrologia 2020, 52, e13590–e13605. [Google Scholar] [CrossRef] [PubMed]







| Proteins | Doxorubicin | EFD | Amino Acid Residues Involved in the Binding Pocket Interactions |
|---|---|---|---|
| BAX | −9.0 | −7.9 | ILE(A:133), ARG(A:134), GLN(A:32). LYS(A:128), GLU(A:41), LEU(A:125), PRO(A:130), ALA(A:35), MET(A:38) |
| BCL-2 | −8.5 | −8.1 | ARG(A:129), GLY(A:8), TYR(A:120), GLU(A:9), PHE(A:128), LEU(A:11), VAL(A:10), PRO(A:125) |
| NF-κB | −2.0 | −1.9 | ASP(B:129), PRO(B:127), LYS(B:128) |
| P53 | −7.6 | −8.5 | THR(A:329), PHE(A:328), LEU(A:330), ASN(A:345), HOH(A:1004), HOH(A:1010), ARG(A:342), PHE(A:338), ILE(A:332),PHE (A:338) |
| Groups | Weight of Whole Epididymis (g) | Weight of Both Testes (g) | Weight of Prostate (g) | Final Body Weight (g) | ROW |
|---|---|---|---|---|---|
| Control | 0.459 ± 0.007 bc | 2.25 ± 0.12 b | 0.385 ± 0.03 d | 213 ± 8.0 c | 0.180 ± 0.006 c |
| Vehicle (10 % DMSO) | 0.456 ± 0.021 b | 2.23 ± 0.18 b | 0.379 ± 0.02 d | 208 ± 11 b | 0.182 ± 0.008 c |
| BPA (50 mg/kg) | 0.334 ± 0.007 a | 1.68 ± 0.29 a | 0.478 ± 0.08 a | 174 ± 13.0 a | 0.274 ± 0.019 a |
| EFD (10 mg/kg) | 0.468 ± 0.056 c | 2.32 ± 0.18 c | 0.388 ± 0.06 d | 221 ± 9.0 d | 0.175 ± 0.008 d |
| BPA + EFD (10 mg/kg) | 0.462 ± 0.063 bc | 2.28 ± 0.11 bc | 0.404 ± 0.05 c | 221 ± 8.0 d | 0.182 ± 0.008 c |
| BPA + EFD (05 mg/kg) | 0.454 ± 0.043 b | 2.26 ± 0.17 b | 0.429 ± 0.04 b | 215 ± 10.0 c | 0.199 ± 0.010 b |
| Groups | RBCs (×106)/µL | WBCs (×103)/µL | Platelets (×103)/µL | Hb (g/dL) | ESR (mm/h) |
|---|---|---|---|---|---|
| Control | 5.93 ± 0.26 d | 3.93 ± 0.32 c | 514.81 ± 4.28 e | 12.33 ± 0.79 de | 3.97 ± 0.25 d |
| Vehicle (10 % DMSO) | 6.08 ± 0.28 e | 3.81 ± 0.45 d | 499.81 ± 4.58 d | 12.45 ± 0.91 e | 4.02 ± 0.45 d |
| BPA (50 mg/kg) | 3.88 ± 0.25 a | 7.37 ± 0.35 a | 308.67 ± 2.51 a | 6.57 ± 0.12 d | 9.19 ± 0.61 a |
| EFD (10 mg/kg) | 5.83 ± 0.19 cd | 4.17 ± 0.11 bc | 489.4 ± 1.51 d | 12.13 ± 0.47 d | 4.13 ± 0.03 cd |
| BPA + EFD (10 mg/kg) | 5.51 ± 0.12 c | 4.32 ± 0.14 bc | 451.2 ± 1.71 c | 11.26 ± 0.71 c | 4.31 ± 0.072 c |
| BPA + EFD (05 mg/kg) | 5.12 ± 0.31 b | 4.85 ± 0.17 b | 405.1 ± 1.91 b | 10.12 ± 0.25 b | 5.12 ± 0.101 b |
| Groups | Testosterone (ng/mL) | FSH (mIU/mL) | LH (mIU/mL) | Estradiol (pg/mL) |
|---|---|---|---|---|
| Control | 4.41 ± 0.12 d | 11.27 ± 0.52 e | 3.14 ± 0.23 d | 18.02 ± 0.94 e |
| Vehicle (10 % DMSO) | 4.33 ± 0.19 d | 10.93 ± 0.75 d | 3.29 ± 0.33 d | 19.14 ± 1.17 d |
| BPA (50 mg/kg) | 1.47 ± 0.16 a | 5.71 ± 0.31 a | 1.41 ± 0.14 a | 26.19 ± 2.16 a |
| EFD (10 mg/kg) | 4.23 ± 0.15 c | 10.59 ± 0.18 cd | 3.14 ± 0.09 d | 19.44 ± 0.41 d |
| BPA + EFD (10 mg/kg) | 4.02 ± 0.09 bc | 10.12 ± 0.27 c | 2.90 ± 0.13 c | 21.07 ± 1.17 c |
| BPA + EFD (05 mg/kg) | 3.81 ± 0.16 b | 8.62 ± 0.36 b | 2.49 ± 0.12 b | 22.82 ± 0.88 b |
| Groups | CAT (U/min) | POD (U/min) | SOD (U/min) | GSH (μM/mg Protein) |
|---|---|---|---|---|
| Control | 3.77 ± 0.12 d | 9.14 ± 0.63 d | 18.02 ± 1.54 e | 24.81 ± 3.14 f |
| Vehicle (10 % DMSO) | 3.83 ± 0.09 d | 9.29 ± 0.39 d | 18.14 ± 1.37 e | 23.68 ± 2.91 e |
| BPA (50 mg/kg) | 1.71 ± 0.07 a | 3.41 ± 0.14 a | 8.19 ± 1.56 a | 12.71 ± 2.84 a |
| EFD (10 mg/kg) | 3.59 ± 0.18 cd | 9.11 ± 0.29 d | 17.14 ± 1.91 d | 22.63 ± 3.19 c |
| BPA + EFD (10 mg/kg) | 3.27 ± 0.27 c | 7.99 ± 0.43 c | 16.67 ± 2.45 c | 21.67 ± 2.14 c |
| BPA + EFD (05 mg/kg) | 3.02 ± 0.21 b | 7.01 ± 0.21 b | 14.54 ± 1.58 b | 19.22 ± 2.43 b |
| Properties | Parameters | EFD |
|---|---|---|
| Physicochemical properties | MW (g/mol) | 428.73 |
| Rotatable bonds | 0 | |
| HBA | 1 | |
| HBD | 1 | |
| Fraction Csp3 | 1.00 | |
| TPSA | 20.23 | |
| Lipophilicity Log Po/w | iLOGP | 4.84 |
| XLOGP3 | 9.94 | |
| MLOGP | 7.07 | |
| Consensus | 7.30 |
| Properties | Parameters | EFD |
|---|---|---|
| Absorption | Water Solubility | −6.49 |
| Caco permeability (cm/s) | 1.389 | |
| GI | 100 | |
| Log Kp (Skin permeation) cm/s | −2.716 | |
| P-gp substrate | No | |
| Distribution | BBB | 0.72 |
| CNS permeation (Log PS) | −1.673 | |
| VD (human) | 0.037 | |
| Metabolism | CYP1A2 inhibitor | No |
| CYP2C19 inhibitor | No | |
| CYP2C9 inhibitor | No | |
| CYP2D6 inhibitor | No | |
| CYP3A4 inhibitor | No | |
| Excretion | Total Clearance (log mL/min/kg) | 0.023 |
| Renal OCT2 substrate | No | |
| Toxicity | AMES toxicity | No |
| hERG I inhibitor | No | |
| hERG II inhibitor | No | |
| Hepatotoxicity | No | |
| Skin Sensitization | No | |
| T. Pyriformis toxicity | 0.354 | |
| Minnow toxicity | −2.303 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majid, M.; Farhan, A.; Baig, M.W.; Khan, M.T.; Kamal, Y.; Hassan, S.S.u.; Bungau, S.; Haq, I.-u. Ameliorative Effect of Structurally Divergent Oleanane Triterpenoid, 3-Epifriedelinol from Ipomoea batatas against BPA-Induced Gonadotoxicity by Targeting PARP and NF-κB Signaling in Rats. Molecules 2023, 28, 290. https://doi.org/10.3390/molecules28010290
Majid M, Farhan A, Baig MW, Khan MT, Kamal Y, Hassan SSu, Bungau S, Haq I-u. Ameliorative Effect of Structurally Divergent Oleanane Triterpenoid, 3-Epifriedelinol from Ipomoea batatas against BPA-Induced Gonadotoxicity by Targeting PARP and NF-κB Signaling in Rats. Molecules. 2023; 28(1):290. https://doi.org/10.3390/molecules28010290
Chicago/Turabian StyleMajid, Muhammad, Anam Farhan, Muhammad Waleed Baig, Muhammad Tariq Khan, Yousaf Kamal, Syed Shams ul Hassan, Simona Bungau, and Ihsan-ul Haq. 2023. "Ameliorative Effect of Structurally Divergent Oleanane Triterpenoid, 3-Epifriedelinol from Ipomoea batatas against BPA-Induced Gonadotoxicity by Targeting PARP and NF-κB Signaling in Rats" Molecules 28, no. 1: 290. https://doi.org/10.3390/molecules28010290
APA StyleMajid, M., Farhan, A., Baig, M. W., Khan, M. T., Kamal, Y., Hassan, S. S. u., Bungau, S., & Haq, I. -u. (2023). Ameliorative Effect of Structurally Divergent Oleanane Triterpenoid, 3-Epifriedelinol from Ipomoea batatas against BPA-Induced Gonadotoxicity by Targeting PARP and NF-κB Signaling in Rats. Molecules, 28(1), 290. https://doi.org/10.3390/molecules28010290