Alkyl Levulinates and 2-Methyltetrahydrofuran: Possible Biomass-Based Solvents in Palladium-Catalyzed Aminocarbonylation
Abstract
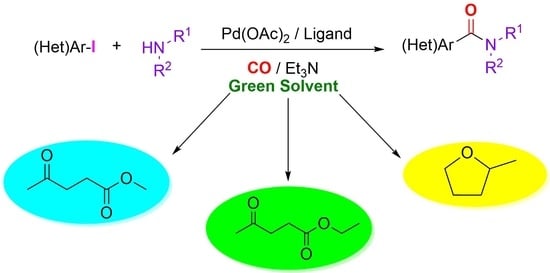
:1. Introduction
2. Results and Discussion
2.1. Optimization Study
2.2. Extending the Scope of Amine Nucleophiles
2.3. Extending the Scope of Substrates
3. Materials and Methods
3.1. Compounds and Solvents
3.2. Aminocarbonylation Reaction under Atmospheric Pressure of CO
3.3. Aminocarbonylation Reaction under High Pressure of CO
3.4. Analytical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Clark, J.H. Green and Sustainable Chemistry: An Introduction. In Green and Sustainable Medicinal Chemistry: Methods, Tools and Strategies for the 21st Century Pharmaceutical Industry; Summerton, L., Sneddon, H.F., Jones, L.C., Clark, J.H., Eds.; The Royal Society of Chemistry: London, UK, 2016; p. 5. Available online: www.rsc.org (accessed on 1 October 2016).
- Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Sherwood, J. Opportunities for Bio-Based Solvents Created as Petrochemical and Fuel Products Transition towards Renewable Resources. Int. J. Mol. Sci. 2015, 16, 17101–17159. [Google Scholar] [CrossRef] [PubMed]
- Global Solvents Industry. Available online: https://www.globenewswire.com/news-release/2020/07/10/2060660/0/en/Global-Solvents-Industry.html (accessed on 28 December 2022).
- Solvents Market by Type (Alcohols, Ketones, Esters, Glycol Ethers, Aromatic, Aliphatic), Application (Paints Coatings, Polymer Manufacturing, Printing Inks), and Region-Global Forecast to 2025. Available online: https://www.marketsandmarkets.com/Market-Reports/solvent-market-1325.html (accessed on 28 December 2022).
- Lomba, L.; Muñiz, S.; Pino, M.R.; Navarro, E.; Giner, B. Ecotoxicity studies of the levulinate ester series. Ecotoxicology 2014, 23, 1484–1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventura, S.P.M.; de Morais, P.; Coelho, J.A.S.; Sintra, T.; Coutinho, J.A.P.; Afonso, C.A.M. Evaluating the toxicity of biomass derived platform chemicals. Green Chem. 2016, 18, 4733–4742. [Google Scholar] [CrossRef]
- Jordan, A.; Hall, C.G.J.; Thorp, L.R.; Sneddon, H.F. Replacement of Less-Preferred Dipolar Aprotic and Ethereal Solvents in Synthetic Organic Chemistry with More Sustainable Alternatives. Chem. Rev. 2022, 122, 6749–6794. [Google Scholar] [CrossRef] [PubMed]
- Pace, V.; Hoyos, P.; Castoldi, L.; De María, P.D.; Alcántara, A.R. 2-Methyltetrahydrofuran (2-MeTHF): A Biomass-Derived Solvent with Broad Application in Organic Chemistry. Chemsuschem 2012, 5, 1369–1379. [Google Scholar] [CrossRef]
- Bijoy, R.; Agarwala, P.; Roy, L.; Thorat, B.N. Unconventional Ethereal Solvents in Organic Chemistry: A Perspective on Applications of 2-Methyltetrahydrofuran, Cyclopentyl Methyl Ether, and 4-Methyltetrahydropyran. Org. Process. Res. Dev. 2021, 26, 480–492. [Google Scholar] [CrossRef]
- Alfonsi, K.; Colberg, J.; Dunn, P.J.; Fevig, T.; Jennings, S.; Johnson, T.A.; Kleine, H.P.; Knight, C.; Nagy, M.A.; Perry, D.A.; et al. Green chemistry tools to influence a medicinal chemistry and research chemistry based organisation. Green Chem. 2007, 10, 31–36. [Google Scholar] [CrossRef]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; McElroy, C.R.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process. 2016, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Prat, D.; Pardigon, O.; Flemming, H.-W.; Letestu, S.; Ducandas, V.; Isnard, P.; Guntrum, E.; Senac, T.; Ruisseau, S.; Cruciani, P.; et al. Sanofi’s Solvent Selection Guide: A Step Toward More Sustainable Processes. Org. Process. Res. Dev. 2013, 17, 1517–1525. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 Selection Guide of Classical- and Less Classical-solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef]
- Capello, C.; Fischer, U.; Hungerbühler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice. Available online: https://www.acs.org/content/acs/en/greenchemistry/principles/12-principles-of-green-chemistry.html (accessed on 10 October 2022).
- Lei, Y.; Wan, Y.; Li, G.; Zhou, X.-Y.; Gu, Y.; Feng, J.; Wang, R. Palladium supported on an amphiphilic porous organic polymer: A highly efficient catalyst for aminocarbonylation reactions in water. Mater. Chem. Front. 2017, 1, 1541–1549. [Google Scholar] [CrossRef]
- Qureshi, Z.S.; Revankar, S.A.; Khedkar, M.V.; Bhanage, B. Aminocarbonylation of aryl iodides with primary and secondary amines in aqueous medium using polymer supported palladium-N-heterocyclic carbene complex as an efficient and heterogeneous recyclable catalyst. Catal. Today 2012, 198, 148–153. [Google Scholar] [CrossRef]
- Khedkar, M.V.; Sasaki, T.; Bhanage, B.M. Immobilized Palladium Metal-Containing Ionic Liquid-Catalyzed Alkoxycarbonylation, Phenoxycarbonylation, and Aminocarbonylation Reactions. ACS Catal. 2013, 3, 287–293. [Google Scholar] [CrossRef]
- Wójcik, P.; Rosar, V.; Gniewek, A.; Milani, B.; Trzeciak, A. In situ generated Pd(0) nanoparticles stabilized by bis(aryl)acenaphthenequinone diimines as catalysts for aminocarbonylation reactions in water. J. Mol. Catal. A Chem. 2016, 425, 322–331. [Google Scholar] [CrossRef] [Green Version]
- Suzuka, T.; Sueyoshi, H.; Ogihara, K. Recyclable Polymer-Supported Terpyridine–Palladium Complex for the Tandem Aminocarbonylation of Aryl Iodides to Primary Amides in Water Using NaN3 as Ammonia Equivalent. Catalysts 2017, 7, 107. [Google Scholar] [CrossRef] [Green Version]
- Mart, M.; Tylus, W.; Trzeciak, A. Pd/DNA as a highly active and recyclable catalyst for aminocarbonylation and hydroxycarbonylation in water: The effect of Mo(CO)6 on the reaction course. Mol. Catal. 2019, 462, 28–36. [Google Scholar] [CrossRef]
- Pace, V.; Castoldi, L.; Alcántara, A.R.; Holzer, W. Highly efficient and environmentally benign preparation of Weinreb amides in the biphasic system 2-MeTHF/water. RSC Adv. 2013, 3, 10158–10162. [Google Scholar] [CrossRef]
- Wu, X.; Ekegren, J.K.; Larhed, M. Microwave-Promoted Aminocarbonylation of Aryl Iodides, Aryl Bromides, and Aryl Chlorides in Water. Organometallics 2006, 25, 1434–1439. [Google Scholar] [CrossRef]
- Bhanage, B.M.; Tambade, P.J.; Patil, Y.P.; Bhanushali, M.J. Pd(OAc)2-Catalyzed Aminocarbonylation of Aryl Iodides with Aromatic or Aliphatic Amines in Water. Synthesis 2008, 2008, 2347–2352. [Google Scholar] [CrossRef]
- Tambade, P.J.; Patil, Y.P.; Qureshi, Z.S.; Dhake, K.P.; Bhanage, B.M. Pd(OAc)2-Catalyzed Carbonylative Coupling of Aryl Iodide with Ortho-Haloamines in Water. Synth. Commun. 2011, 42, 176–185. [Google Scholar] [CrossRef]
- Ács, P.; Takács, A.; Szilágyi, A.; Wölfling, J.; Schneider, G.; Kollár, L. The synthesis of 13α-androsta-5,16-diene derivatives with carboxylic acid, ester and carboxamido functionalities at position-17 via palladium-catalyzed carbonylation. Steroids 2009, 74, 419–423. [Google Scholar] [CrossRef]
- Beller, M.; Wu, X.F. Transition Metal Catalyzed Carbonylation Reactions; Springer: Berlin/Heidelberg, Germany, 2013; p. 1. [Google Scholar]
- Santoro, S.; Ferlin, F.; Luciani, L.; Ackermann, L.; Vaccaro, L. Biomass-derived solvents as effective media for cross-coupling reactions and C–H functionalization processes. Green Chem. 2017, 19, 1601–1612. [Google Scholar] [CrossRef]
- Takács, A.; Farkas, R.; Kollár, L. High-yielding synthesis of 2-arylacrylamides via homogeneous catalytic aminocarbonylation of α-iodostyrene and α,α′-diiodo-1,4-divinylbenzene. Tetrahedron 2008, 64, 61–66. [Google Scholar] [CrossRef]
- Takács, A.; Farkas, R.; Petz, A.; Kollár, L. Synthesis of 2-naphthylacrylamides and 2-naphthylacrylates via homogeneous catalytic carbonylation of 1-iodo-1-naphthylethene derivatives. Tetrahedron 2009, 65, 4795–4800. [Google Scholar] [CrossRef]
- Gergely, M.; Farkas, R.; Takács, A.; Petz, A.; Kollár, L. Synthesis of N-picolylcarboxamides via palladium-catalysed aminocarbonylation of iodobenzene and iodoalkenes. Tetrahedron 2014, 70, 218–224. [Google Scholar] [CrossRef]
- Ács, P.; Takács, A.; Kiss, M.; Pálinkás, N.; Mahó, S.; Kollár, L. Systematic investigation on the synthesis of androstane-based 3-, 11- and 17-carboxamides via palladium-catalyzed aminocarbonylation. Steroids 2011, 76, 280–290. [Google Scholar] [CrossRef]
- Takács, A.; Ács, P.; Farkas, R.; Kokotos, G.; Kollár, L. Homogeneous catalytic aminocarbonylation of 1-iodo-1-dodecene. The facile synthesis of odd-number carboxamides via palladium-catalysed aminocarbonylation. Tetrahedron 2008, 64, 9874–9878. [Google Scholar] [CrossRef]
- Takács, A.; Ács, P.; Berente, Z.; Wölfling, J.; Schneider, G.; Kollár, L. Novel 13β- and 13α-d-homo steroids: 17a-carboxamido-d-homoestra-1,3,5(10),17-tetraene derivatives via palladium-catalyzed aminocarbonylations. Steroids 2010, 75, 1075–1081. [Google Scholar] [CrossRef]
- Kégl, T.R.; Mika, L.T.; Kégl, T. 27 Years of Catalytic Carbonylative Coupling Reactions in Hungary (1994–2021). Molecules 2022, 27, 460. [Google Scholar] [CrossRef]
- Mikle, G.; Bede, F.; Kollár, L. Synthesis of N-picolylcarboxamides in aminocarbonylation. Tetrahedron 2021, 88, 132128. [Google Scholar] [CrossRef]
- Szuroczki, P.; Boros, B.; Kollár, L. Efficient synthesis of alkynyl amides via aminocarbonylation of iodoalkynes. Tetrahedron 2018, 74, 6129–6136. [Google Scholar] [CrossRef]
- Gergely, M.; Kollár, L. Aminothiazoles and aminothiadiazoles as nucleophiles in aminocarbonylation of iodobenzene derivatives. Tetrahedron 2018, 74, 2030–2040. [Google Scholar] [CrossRef]
- Mikle, G.; Skoda-Földes, R.; Kollár, L. Amino- and azidocarbonylation of iodoalkenes. Tetrahedron 2021, 100, 132495. [Google Scholar] [CrossRef]
- Ismael, A.; Gevorgyan, A.; Skrydstrup, T.; Bayer, A. Renewable Solvents for Palladium-Catalyzed Carbonylation Reactions. Org. Process. Res. Dev. 2020, 24, 2665–2675. [Google Scholar] [CrossRef]
- Fodor, D.; Kégl, T.; Tukacs, J.M.; Horváth, A.K.; Mika, L.T. Homogeneous Pd-Catalyzed Heck Coupling in γ-Valerolactone as a Green Reaction Medium: A Catalytic, Kinetic, and Computational Study. ACS Sustain. Chem. Eng. 2020, 8, 9926–9936. [Google Scholar] [CrossRef]
- Marosvölgyi-Haskó, D.; Lengyel, B.; Tukacs, J.M.; Kollár, L.; Mika, L.T. Application of γ-Valerolactone as an Alternative Biomass-Based Medium for Aminocarbonylation Reactions. ChemPlusChem 2016, 81, 1224–1229. [Google Scholar] [CrossRef]
- Tukacs, J.M.; Marton, B.; Albert, E.; Tóth, I.; Mika, L.T. Palladium-catalyzed aryloxy- and alkoxycarbonylation of aromatic iodides in γ-valerolactone as bio-based solvent. J. Organomet. Chem. 2020, 923, 121407. [Google Scholar] [CrossRef]
- Ojima, I.; Commandeur, C.; Chiou, W.-H. Amidocarbonylation, Cyclohydrocarbonylation and Related Reactions. In Comprehensive Organic Chemistry III, (COMC-III), 3rd ed.; Micheal, D., Mingos, P., Crabtree, R.H., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2007; Volume 13, pp. 511–555. [Google Scholar]
- Tomé, V.A.; Calvete, M.J.F.; Vinagreiro, C.S.; Aroso, R.T.; Pereira, M.M. A New Tool in the Quest for Biocompatible Phthalocyanines: Palladium Catalyzed Aminocarbonylation for Amide Substituted Phthalonitriles and Illustrative Phthalocyanines Thereof. Catalysts 2018, 8, 480. [Google Scholar] [CrossRef] [Green Version]
- Bousfield, T.W.; Pearce, K.P.R.; Nyamini, S.B.; Angelis-Dimakis, A.; Camp, J.E. Synthesis of amides from acid chlorides and amines in the bio-based solvent Cyrene™. Green Chem. 2019, 21, 3675–3681. [Google Scholar] [CrossRef]
- Yan, K.; Yang, Y.; Chai, J.; Lu, Y. Catalytic reactions of gamma-valerolactone: A platform to fuels and value-added chemicals. Appl. Catal. B Environ. 2015, 179, 292–304. [Google Scholar] [CrossRef]
- Pastore, C.; D’Ambrosio, V. Intensification of Processes for the Production of Ethyl Levulinate Using AlCl3·6H2O. Energies 2021, 14, 1273. [Google Scholar] [CrossRef]
- Lei, P.; Mu, Y.; Wang, Y.; Wang, Y.; Ma, Z.; Feng, J.; Liu, X.; Szostak, M. Green Solvent Selection for Suzuki–Miyaura Coupling of Amides. ACS Sustain. Chem. Eng. 2020, 9, 552–559. [Google Scholar] [CrossRef]
- Yan, K.; Jarvis, C.; Gu, J.; Yan, Y. Production and catalytic transformation of levulinic acid: A platform for speciality chemicals and fuels. Renew. Sustain. Energy Rev. 2015, 51, 986–997. [Google Scholar] [CrossRef]
- Gao, W.; Wu, G.; Zhu, X.; Akhtar, M.A.; Lin, G.; Huang, Y.; Zhang, S.; Zhang, H. Production of methyl levulinate from cellulose over cobalt disulfide: The importance of the crystal facet (111). Bioresour. Technol. 2021, 347, 126436. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.F.L.; Mariano, A.P.; Filho, R.M. Economic potential of 2-methyltetrahydrofuran (MTHF) and ethyl levulinate (EL) produced from hemicelluloses-derived furfural. Biomass- Bioenergy 2018, 119, 492–502. [Google Scholar] [CrossRef]
- Xu, W.; Chen, X.; Guo, H.; Li, H.; Zhang, H.; Xiong, L.; Chen, X. Conversion of levulinic acid to valuable chemicals: A review. J. Chem. Technol. Biotechnol. 2021, 96, 3009–3024. [Google Scholar] [CrossRef]
- Sherwood, J.; Clark, J.H.; Fairlamb, I.J.S.; Slattery, J.M. Solvent effects in palladium catalysed cross-coupling reactions. Green Chem. 2019, 21, 2164–2213. [Google Scholar] [CrossRef] [Green Version]
- Hao, W.; Xu, Z.; Zhou, Z.; Cai, M. Recyclable Heterogeneous Palladium-Catalyzed Cyclocarbonylation of 2-Iodoanilines with Acyl Chlorides in the Biomass-Derived Solvent 2-Methyltetrahydrofuran. J. Org. Chem. 2020, 85, 8522–8532. [Google Scholar] [CrossRef]
- Kadam, A.; Nguyen, M.; Kopach, M.; Richardson, P.; Gallou, F.; Wan, Z.-K.; Zhang, W. Comparative performance evaluation and systematic screening of solvents in a range of Grignard reactions. Green Chem. 2013, 15, 1880–1888. [Google Scholar] [CrossRef]
- Mondal, M.; Bora, U. Eco-friendly Suzuki–Miyaura coupling of arylboronic acids to aromatic ketones catalyzed by the oxime-palladacycle in biosolvent 2-MeTHF. New J. Chem. 2016, 40, 3119–3123. [Google Scholar] [CrossRef]
- Santoro, S.; Marrocchi, A.; Lanari, D.; Ackermann, L.; Vaccaro, L. Towards Sustainable C−H Functionalization Reactions: The Emerging Role of Bio-Based Reaction Media. Chem.-A Eur. J. 2018, 24, 13383–13390. [Google Scholar] [CrossRef] [PubMed]
- Přibylka, A.; Krchňák, V.; Schütznerová, E. Environmentally Friendly SPPS II: Scope of Green Fmoc Removal Protocol Using NaOH and Its Application for Synthesis of Commercial Drug Triptorelin. J. Org. Chem. 2020, 85, 8798–8811. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, S.; Castoldi, L.; Murgia, I.; Senatore, R.; Mazzeo, E.; Wackerlig, J.; Urban, E.; Langer, T.; Pace, V. Recent advancements on the use of 2-methyltetrahydrofuran in organometallic chemistry. 2016, 148, 37–48. [CrossRef] [Green Version]
- Sardana, M.; Bergman, J.; Ericsson, C.; Kingston, L.P.; Schou, M.; Dugave, C.; Audisio, D.; Elmore, C.S. Visible-Light-Enabled Aminocarbonylation of Unactivated Alkyl Iodides with Stoichiometric Carbon Monoxide for Application on Late-Stage Carbon Isotope Labeling. J. Org. Chem. 2019, 84, 16076–16085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barré, A.; Ţînţaş, M.-L.; Alix, F.; Gembus, V.; Papamicaël, C.; Levacher, V. Palladium-Catalyzed Carbonylation of (Hetero)Aryl, Alkenyl and Allyl Halides by Means of N-Hydroxysuccinimidyl Formate as CO Surrogate. J. Org. Chem. 2015, 80, 6537–6544. [Google Scholar] [CrossRef] [PubMed]
- Nathel, N.F.F.; Kim, J.; Hie, L.; Jiang, X.; Garg, N.K. Nickel-Catalyzed Amination of Aryl Chlorides and Sulfamates in 2-Methyl-THF. ACS Catal. 2014, 4, 3289–3293. [Google Scholar] [CrossRef] [Green Version]
- Skoda-Foldes, R. Palladium-Catalyzed Aminocarbonylation of Iodoalkenes and Iodoarenes. Lett. Org. Chem. 2010, 7, 621–633. [Google Scholar] [CrossRef]
- Marosvölgyi-Haskó, D.; Kégl, T.; Kollár, L. Substituent effects in aminocarbonylation of para -substituted iodobenzenes. Tetrahedron 2016, 72, 7509–7516. [Google Scholar] [CrossRef]
- Takacs, A.; Petz, A.; Jakab, B.; Kollár, L. Aminocarbonylation of 2-Iodothiophene: High-Yielding Synthesis of Thiophen-2-yl-glyoxylamides. Lett. Org. Chem. 2007, 4, 590–594. [Google Scholar] [CrossRef]
- Takács, A.; Jakab, B.; Petz, A.; Kollár, L. Homogeneous catalytic aminocarbonylation of nitrogen-containing iodo-heteroaromatics. Synthesis of N-substituted nicotinamide related compounds. Tetrahedron 2007, 63, 10372–10378. [Google Scholar] [CrossRef]
- Takács, A.; Marosvölgyi-Haskó, D.; Kabak-Solt, Z.; Damas, L.; Rodrigues, F.M.; Carrilho, R.M.; Pineiro, M.; Pereira, M.M.; Kollár, L. Functionalization of indole at C-5 or C-7 via palladium-catalysed double carbonylation. A facile synthesis of indole ketocarboxamides and carboxamide dimers. Tetrahedron 2016, 72, 247–256. [Google Scholar] [CrossRef]
- Kollár, L.; Erdélyi, A.; Rasheed, H.; Takács, A. Selective Synthesis of N-Acylnortropane Derivatives in Palladium-Catalysed Aminocarbonylation. Molecules 2021, 26, 1813. [Google Scholar] [CrossRef] [PubMed]
- Kollár, L.; Takács, A. Novel synthesis of 3-carboxamidolactam derivatives via palladium-catalysed aminocarbonylation. Tetrahedron 2018, 74, 6116–6128. [Google Scholar] [CrossRef]
- Baburajan, P.; Elango, K.P. Co2(CO)8 as a convenient in situ CO source for the direct synthesis of benzamides from aryl halides (Br/I) via aminocarbonylation. Tetrahedron Lett. 2014, 55, 1006–1010. [Google Scholar] [CrossRef]
- Gockel, S.N.; Hull, K.L. Chloroform as a Carbon Monoxide Precursor: In or Ex Situ Generation of CO for Pd-Catalyzed Aminocarbonylations. Org. Lett. 2015, 17, 3236–3239. [Google Scholar] [CrossRef]
- Wang, P.; Yang, J.; Sun, K.; Neumann, H.; Beller, M. A general synthesis of aromatic amides via palladium-catalyzed direct aminocarbonylation of aryl chlorides. Org. Chem. Front. 2022, 9, 2491–2497. [Google Scholar] [CrossRef]









 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Solvent | Ligand | Temp. | pco | Conv.(b) | Ratio of Products (b) | |
| [°C] | [bar] | [%] | Amide | Ketoamide | |||
| 1 | DMF | PPh₃ | 50 | 1 | 71 | 28 | 72 |
| 2 | DMF | PPh₃ | 70 | 1 | 74 | 67 | 33 |
| 3 | DMF | PPh₃ | 50 | 40 | 79 | 3 | 97 |
| 4 | DMF | XantPhos | 50 | 1 | 100 | 88 | 12 |
| 5 | MetLev | PPh₃ | 50 | 1 | 29 | 59 | 41 |
| 6 | MetLev | PPh₃ | 70 | 1 | 62 | 88 | 12 |
| 7 | MetLev | PPh₃ | 50 | 40 | 63 | 5 | 95 |
| 8 | MetLev | XantPhos | 50 | 1 | 99 | 100 | 0 |
| 9 | EtLev | PPh₃ | 50 | 1 | 46 | 68 | 32 |
| 10 | EtLev | PPh₃ | 70 | 1 | 69 | 91 | 9 |
| 11 | EtLev | PPh₃ | 50 | 40 | 78 | 8 | 92 |
| 12 | EtLev | XantPhos | 50 | 1 | 98 | 100 | 0 |
| 13 | 2-MeTHF | PPh₃ | 50 | 1 | 9 | 84 | 16 |
| 14 | 2-MeTHF | PPh₃ | 70 | 1 | 20 | 82 | 18 |
| 15 | 2-MeTHF | PPh₃ | 50 | 40 | 31 | 14 | 86 |
| 16 | 2-MeTHF | XantPhos | 50 | 1 | 87 | 100 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uzunlu, N.; Pongrácz, P.; Kollár, L.; Takács, A. Alkyl Levulinates and 2-Methyltetrahydrofuran: Possible Biomass-Based Solvents in Palladium-Catalyzed Aminocarbonylation. Molecules 2023, 28, 442. https://doi.org/10.3390/molecules28010442
Uzunlu N, Pongrácz P, Kollár L, Takács A. Alkyl Levulinates and 2-Methyltetrahydrofuran: Possible Biomass-Based Solvents in Palladium-Catalyzed Aminocarbonylation. Molecules. 2023; 28(1):442. https://doi.org/10.3390/molecules28010442
Chicago/Turabian StyleUzunlu, Nuray, Péter Pongrácz, László Kollár, and Attila Takács. 2023. "Alkyl Levulinates and 2-Methyltetrahydrofuran: Possible Biomass-Based Solvents in Palladium-Catalyzed Aminocarbonylation" Molecules 28, no. 1: 442. https://doi.org/10.3390/molecules28010442
APA StyleUzunlu, N., Pongrácz, P., Kollár, L., & Takács, A. (2023). Alkyl Levulinates and 2-Methyltetrahydrofuran: Possible Biomass-Based Solvents in Palladium-Catalyzed Aminocarbonylation. Molecules, 28(1), 442. https://doi.org/10.3390/molecules28010442