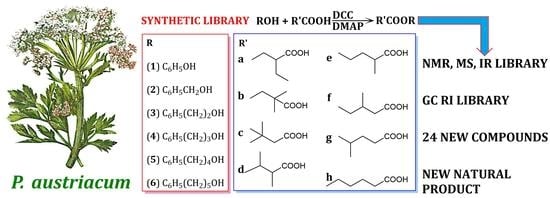
3.6. Synthesis of Esters
A solution of the appropriate alcohol (phenol (
1), phenylmethanol (
2), 2-phenylethanol (
3), 3-phenylpropan-1-ol (
4), 4-phenylbutan-1-ol (
5), and 5-phenylpentan-1-ol (
6)), carboxylic acid (1.1 eq; 2-ethylbutanoic (
a), 2,2-dimethylbutanoic (
b), 3,3-dimethylbutanoic (
c), 2,3-dimethylbutanoic (
d), 2-methylpentanoic (
e), 3-methylpentanoic (
f), 4-methylpentanoic (
g), and hexanoic acid (
h)), DMAP (0.3 eq) and DCC (1.1 eq) in 30 mL of dry DCM was stirred overnight, at room temperature, in a round bottom flask equipped with a CaCl
2 guard tube. The precipitated urea was filtered off and the filtrate was concentrated under a vacuum. The resulting residue was purified by “dry-flash” chromatography using mixtures of hexane and Et
2O of increasing polarity for elution. Esters were washed from the column with 10% (
v/
v) Et
2O in hexane. The purity of the ester fractions was checked by TLC and GC-MS. The yield of the esterification step, spectral data (NMR, MS, and IR), and assignments of
1H and
13C signals for the synthesized esters are given below and in the
Supplementary Materials (Figures S1–S144).
Phenyl 2-ethylbutanoate (1a), Yield: 48%; IR (cm−1) 2964, 2935, 2877, 1753, 1594, 1493, 1458, 1384, 1372, 1266, 1193, 1157, 1110, 1069, 1024, 927, 899, 823, 744, 688; 1H NMR (400 MHz, CDCl3) 7.40–7.34 (2H, multiplet, H-3′ and H-5′), 7.26–7.17 (1H, multiplet, H-4′), 7.09–7.05 (2H, multiplet, H-2′ and H-6′), 2.4570* (1H, triplet of triplets, J = 8.9, 5.5 Hz, H-2), 1.7813* (1H, doublet of doublets of doublets, J = −13.5, 8.9, 7.4 Hz, H-3b), 1.7804 * (1H, doublet of doublets of doublets, J = −13.5, 8.9, 7.4 Hz, H-5b), 1.6614* (1H, doublet of doublets of doublets, J = −13.5, 7.4, 5.5 Hz, H-3a), 1.6608* (1H, doublet of doublets of doublets, J = −13.5, 7.4, 5.5 Hz, H-5a), 1.0240 * (6H, triplet, J = 7.4 Hz, H-4 and H-6); 13C NMR (101 MHz, CDCl3) 174.70 (C-1), 150.82 (C-1′), 129.37 (C-3′ and C-5′), 125.66 (C-4′), 121.63 (C-2′ and C-6′), 48.96 (C-2), 25.13 (C-3 and C-5), 11.86 (C-4 and C-6); MS (EI), m/z (%) 192 (2), 99 (36), 98 (26), 94 (100), 77 (15), 71 (91), 66 (9), 65 (18), 55 (18), 43 (46), 41 (15). * The values of chemical shift and coupling constants were determined by a simulation of the 1H NMR spectrum (manual iterative full spin analysis).
Phenyl 2,2-dimethylbutanoate (1b), Yield: 52%; IR (cm−1) 2969, 2935, 2877, 1750, 1593, 1494, 1474, 1458, 1389, 1366, 1314, 1234, 1192, 1161, 1108, 1070, 1056, 1008, 930, 912, 871, 831, 781, 737, 688; 1H NMR (400 MHz, CDCl3) 7.40–7.33 (2H, multiplet, H-3′ and H-5′), 7.24–7.18 (1H, multiplet, H-4′), 7.07–7.02 (2H, multiplet, H-2′ and H-6′), 1.73 (2H, quartet, J = 7.5 Hz, H-3), 1.31 (6H, singlet, H-5 and H-6), 0.98 (3H, triplet, J = 7.5 Hz, H-4); 13C NMR (101 MHz, CDCl3) 176.52 (C-1), 151.06 (C-1′), 129.33 (C-3′ and C-5′), 125.56 (C-4′), 121.55 (C-2′ and C-6′), 42.95 (C-2), 33.38 (C-3), 24.68 (C-5 and C-6), 9.36 (C-4); MS (EI), m/z (%) 192 (4), 99 (12), 95 (8), 94 (100), 77 (8), 71 (48), 70 (12), 65 (10), 55 (9), 43 (20), 41 (10).
Phenyl 3,3-dimethylbutanoate (1c), Yield: 70%; IR (cm−1) 2970, 2934, 2875, 1751, 1592, 1494, 1470, 1388, 1360, 1235, 1193, 1162, 1072, 1057, 1009, 932, 870, 830, 737, 688; 1H NMR (400 MHz, CDCl3) 7.40–7.34 (2H, multiplet, H-3′, and H-5′), 7.26–7.17 (1H, multiplet, H-4′), 7.10–7.05 (2H, multiplet, H-2′, and H-6′), 2.44 (2H, singlet, H-2), 1.14 (9H, singlet, H-4–H-6); 13C NMR (101 MHz, CDCl3) 170.73 (C-1), 150.67 (C-1′), 129.37 (C-3′ and C-5′), 125.68 (C-4′), 121.66 (C-2′ and C-6′), 47.82 (C-2), 31.13 (C-3), 29.68 (C-4–C-6); MS (EI), m/z (%) 192 (3), 99 (73), 94 (100), 77 (21), 71 (17), 65 (25), 55 (9), 41 (25).
Phenyl 2,3-dimethylbutanoate (1d), Yield: 66%; IR (cm−1) 2965, 2935, 2875, 1754, 1593, 1493, 1457, 1388, 1369, 1352, 1231, 1196, 1178, 1161, 1110, 1070, 1023, 1003, 924, 848, 749, 688; 1H NMR (400 MHz, CDCl3) 7.40–7.34 (2H, multiplet, H-3′ and H-5′), 7.24–7.19 (1H, multiplet, H-4′), 7.09–7.04 (2H, multiplet, H-2′ and H-6′), 2.49 (1H, pseudo quintet, J = 7.0 Hz, H-2), 2.08 (1H, pseudo octet, J = 7.0 Hz, H-3), 1.25 (3H, doublet, J = 7.0 Hz, H-6), 1.05 (3H, doublet, J = 7.0 Hz, H-4), 1.01 (3H, doublet, J = 7.0 Hz, H-5); 13C NMR (101 MHz, CDCl3) 174.82 (C-1), 150.83 (C-1′), 129.36 (C-3′ and C-5′), 125.64 (C-4′), 121.58 (C-2′ and C-6′), 46.20 (C-2), 31.16 (C-3), 20.70 (C-4), 19.23 (C-5), 13.72 (C-6); MS (EI), m/z (%) 192 (4), 99 (37), 98 (25), 95 (15), 94 (84), 77 (16), 71 (100), 65 (19), 55 (19), 43 (53), 41 (17).
Phenyl 2-methylpentanoate (1e), Yield: 74%; IR (cm−1) 2960, 2934, 2874, 1754, 1593, 1493, 1456, 1379, 1273, 1194, 1160, 1113, 1069, 1050, 1025, 997, 912, 887, 852, 811, 743, 688; 1H NMR (400 MHz, CDCl3) 7.40–7.34 (2H, multiplet, H-3′ and H-5′), 7.26–7.16 (1H, multiplet, H-4′), 7.09–7.05 (2H, multiplet, H-2′ and H-6′), 2.70 (1H, pseudo sextet, J = 6.9 Hz, H-2), 1.85–1.75 (1H, multiplet, Ha-3), 1.59–1.50 (1H, multiplet, Hb-3), 1.49–1.40 (2H, multiplet, H-4), 1.29 (3H, doublet, J = 6.9 Hz, H-6), 0.97 (3H, triplet, J = 7.2, Hz H-5); 13C NMR (101 MHz, CDCl3) 175.33 (C-1), 150.87 (C-1′), 129.36 (C-3′ and C-5′), 125.63 (C-4′), 121.55 (C-2′ and C-6′), 39.44 (C-2), 35.93 (C-3), 20.45 (C-4), 17.00 (C-6), 13.99 (C-5); MS (EI), m/z (%) 192 (4), 99 (30), 98 (33), 94 (100), 77 (15), 71 (96), 69 (12), 65 (21), 55 (12), 43 (51), 41 (18).
Phenyl 3-methylpentanoate (1f), Yield: 80%; IR (cm−1) 2961, 2929, 2876, 1753, 1593, 1493, 1457, 1379, 1314, 1285, 1262, 1234, 1195, 1162, 1145, 1104, 1024, 937, 887, 812, 760, 687; 1H NMR (400 MHz, CDCl3) 7.40–7.34 (2H, multiplet, H-3′ and H-5′), 7.26–7.17 (1H, multiplet, H-4′), 7.10–7.04 (2H, multiplet, H-2′ and H-6′), 2.56 (1H, doublet of doublets, J = 14.8, 6.1 Hz, Ha-2), 2.35 (1H, doublet of doublets, J = 14.8, 8.2 Hz, Hb-2), 2.03 (1H, doublet of doublets of quartets of doublets of doublets, J = 8.2, 7.4, 6.7, 6.1, 5.7 Hz, H-3), 1.47 (1H, doublet of quartets of doublets, J = 13.3, 7.4, 5.7 Hz, Ha-4), 1.33 (1H, pseudo doublet of quintets, J = 13.3, 7.4 Hz, Hb-4), 1.04 (3H, doublet, J = 6.7 Hz, H-6), 0.95 (3H, triplet, J = 7.4 Hz, H-5); 13C NMR (101 MHz, CDCl3) 171.78 (C-1), 150.76 (C-1′), 129.38 (C-3′ and C-5′), 125.69 (C-4′), 121.60 (C-2′ and C-6′), 41.45 (C-2), 32.10 (C-3), 29.37 (C-4), 19.31 (C-6), 11.32 (C-5); MS (EI), m/z (%) 192 (10), 99 (80), 95 (14), 94 (100), 77 (20), 71 (63), 69 (21), 65 (23), 55 (10), 43 (51), 41 (22).
Phenyl 4-methylpentanoate (1g), Yield: 88%; IR (cm−1) 2956, 2929, 2871, 1755, 1593, 1493, 1469, 1387, 1368, 1329, 1269, 1194, 1161, 1141, 1096, 1070, 1024, 1007, 928, 897, 813, 747, 688; 1H NMR (400 MHz, CDCl3) 7.40–7.34 (2H, multiplet, H-3′ and H-5′), 7.24–7.19 (1H, multiplet, H-4′), 7.10–7.04 (2H, multiplet, H-2′ and H-6′), 2.60–2.52 (2H, multiplet, H-2), 1.73–1.61 (3H, overlapping peaks, H-3 and H-4), 0.96 (6H, doublet, J = 6.4 Hz, H-5 and H-6); 13C NMR (101 MHz, CDCl3) 172.50 (C-1), 150.75 (C-1′), 129.38 (C-3′ and C-5′), 125.69 (C-4′), 121.56 (C-2′ and C-6′), 33.72 (C-3), 32.50 (C-2), 27.73 (C-4), 22.25 (C-5 and C-6); MS (EI), m/z (%) 192 (10), 99 (38), 95 (18), 94 (100), 81 (49), 77 (14), 71 (13), 65 (17), 55 (21), 43 (46), 41 (16).
Phenyl hexanoate (1h), Yield: 86%; IR (cm−1) 2957, 2930, 2871, 1759, 1593, 1493, 1456, 1363, 1194, 1161, 1140, 1101, 1070, 1024, 1007, 932, 877, 813, 750, 689; 1H NMR (400 MHz, CDCl3) 7.40–7.34 (2H, multiplet, H-3′ and H-5′), 7.24–7.19 (1H, multiplet, H-4′), 7.10–7.05 (2H, multiplet, H-2′ and H-6′), 2.55 (2H, triplet, J = 7.5 Hz, H-2), 1.76 (2H, quintet, J = 7.5 Hz, H-3), 1.45–1.32 (4H, overlapping peaks, H-4 and H-5), 0.92 (3H, triplet, J = 7.2 Hz, H-6); 13C NMR (101 MHz, CDCl3) 172.23 (C-1), 150.75 (C-1′), 129.38 (C-3′ and C-5′), 125.69 (C-4′), 121.58 (C-2′ and C-6′), 34.37 (C-2), 31.27 (C-4), 24.64 (C-3), 22.33 (C-5), 13.94 (C-6); MS (EI), m/z (%) 192 (9), 99 (46), 95 (10), 94 (100), 77 (12), 71 (28), 65 (16), 55 (13), 43 (46), 41 (14).
Benzyl 2-ethylbutanoate (2a), Yield: 49%; IR (cm−1) 2958, 2930, 2870, 1753, 1593, 1493, 1455, 1362, 1190, 1161, 1140, 1075, 1007, 932, 876, 750, 689; 1H NMR (400 MHz, CDCl3) 7.40–7.29 (5H, multiplet, H-3′–H-7′), 5.13 (2H, singlet, H-1′), 2.2602* (1H, triplet of triplets, J = 8.9, 5.5 Hz, H-2), 1.6412* (1H, doublet of doublets of doublets, J = −13.5, 8.9, 7.4 Hz, H-3b), 1.6403* (1H, doublet of doublets of doublets, J = −13.5, 8.9, 7.4 Hz, H-5b), 1.5412* (1H, doublet of doublets of doublets, J = −13.5, 7.4, 5.5 Hz, H-3a), 1.5408* (1H, doublet of doublets of doublets, J = −13.5, 7.4, 5.5 Hz, H-5a), 0.8839* (6H, triplet, J = 7.4 Hz, H-4 and H-6); 13C NMR (101 MHz, CDCl3) 176.13 (C-1), 136.30 (C-2′), 128.50 (C-4′ and C-6′), 128.12 (C-3′ and C-7′), 128.08 (C-5′), 65.87 (C-1′), 48.92 (C-2), 25.05 (C-3 and C-5), 11.82 (C-4 and C-6); MS (EI), m/z (%) 206 (6), 92 (11), 91 (100), 89 (8), 77 (11), 71 (75), 65 (17), 55 (11), 43 (44), 41 (13). * The values of chemical shift and coupling constants were determined by a simulation of the 1H NMR spectrum (manual iterative full spin analysis).
Benzyl 2,2-dimethylbutanoate (2b), Yield: 51%; IR (cm−1) 2969, 2932, 1727, 1498, 1474, 1455, 1389, 1314, 1238, 1137, 1062, 1029, 1008, 968, 911, 793, 734, 696; 1H NMR (400 MHz, CDCl3) 7.39–7.28 (5H, multiplet, H-3′–H-7′), 5.11 (2H, singlet, H-1′), 1.59 (2H, quartet, J = 7.5 Hz, H-3), 1.18 (6H, singlet, H-5 and H-6), 0.82 (3H, triplet, J = 7.5 Hz, H-4); 13C NMR (101 MHz, CDCl3) 177.80 (C-1), 136.47 (C-2′), 128.46 (C-4′ and C-6′), 127.95 (C-3′ and C-7′), 127.79 (C-5′), 65.96 (C-1′), 42.71 (C-2), 33.36 (C-3), 24.66 (C-5 and C-6), 9.23 (C-4); MS (EI), m/z (%) 206 (7), 92 (9), 91 (100), 89 (6), 77 (8), 71 (80), 65 (16), 55 (6), 43 (46), 41 (11).
Benzyl 3,3-dimethylbutanoate (2c), Yield: 67%; IR (cm−1) 2955, 2932, 2868, 1731, 1498, 1455, 1367, 1321, 1224, 1125, 1045, 996, 891, 737, 696; 1H NMR (400 MHz, CDCl3) 7.38–7.29 (5H, multiplet, H-3′–H-7′), 5.10 (2H, singlet, H-1′), 2.25 (2H, singlet, H-2), 1.02 (9H, singlet, H-4–H-6); 13C NMR (101 MHz, CDCl3) 172.17 (C-1), 136.14 (C-2′), 128.50 (C-4′ and C-6′), 128.26 (C-3′ and C-7′), 128.11 (C-5′), 65.89 (C-1′), 47.93 (C-2), 30.83 (C-3), 29.64 (C-4–C-6); MS (EI), m/z (%) 206 (8), 131 (5), 108 (36), 99 (9), 92 (8), 91 (100), 77 (6), 65 (10), 57 (19), 41 (8).
Benzyl 2,3-dimethylbutanoate (2d), Yield: 65%; IR (cm−1) 2955, 2867, 1733, 1497, 1454, 1365, 1321, 1124, 1045, 999, 737, 695; 1H NMR (400 MHz, CDCl3) 7.39–7.28 (5H, multiplet, H-3′–H-7′), 5.11 (2H, singlet, H-1′), 2.29 (1H, pseudo quintet, J = 7.0 Hz, H-2), 1.94 (1H, pseudo octet, J = 7.0 Hz, H-3), 1.12 (3H, doublet, J = 7.0 Hz, H-6), 0.91 (3H, doublet, J = 7.0 Hz, H-4), 0.89 (3H, doublet, J = 7.0 Hz, H-5); 13C NMR (101 MHz, CDCl3) 176.25 (C-1), 136.24 (C-2′), 128.50 (C-4′ and C-6′), 128.12 (C-3′ and C-7′), 128.08 (C-5′), 65.90 (C-1′), 46.16 (C-2), 31.03 (C-3), 20.71 (C-4), 19.14 (C-5), 13.68 (C-6); MS (EI), m/z (%) 206 (2), 131 (4), 108 (44), 99 (12), 92 (6), 91 (100), 77 (8), 65 (13), 57 (11), 41 (5).
Benzyl 2-methylpentanoate (2e), Yield: 70%; IR (cm−1) 2959, 2933, 2873, 1732, 1498, 1455, 1381, 1350, 1230, 1213, 1171, 1142, 1081, 1028, 1004, 966, 910, 735, 696; 1H NMR (400 MHz, CDCl3) 7.39–7.28 (5H, multiplet, H-3′–H-7′), 5.11 (2H, singlet, H-1′), 2.50 (1H, sextet, J = 7.0 Hz, H-2), 1.72–1.62 (1H, multiplet, Ha-3), 1.46–1.36 (1H, multiplet, Hb-3), 1.35–1.24 (2H, multiplet, H-4), 1.16 (3H, doublet, J = 7.0 Hz, H-6), 0.89 (3H, triplet, J = 7.2 Hz, H-5); 13C NMR (101 MHz, CDCl3) 176.73 (C-1), 136.28 (C-2′), 128.51 (C-4′ and C-6′), 128.07 (C-3′ and C-7′), 128.01 (C-5′), 65.95 (C-1′), 39.32 (C-2), 35.92 (C-3), 20.37 (C-4), 17.01 (C-6), 13.94 (C-5); MS (EI), m/z (%) 206 (2), 164 (5), 108 (13), 99 (5), 92 (13), 91 (100), 77 (6), 71 (18), 65 (11), 43 (18), 41 (7).
Benzyl 3-methylpentanoate (2f), Yield: 75%; IR (cm−1) 2960, 2930, 2876, 1732, 1498, 1456, 1380, 1357, 1285, 1238, 1174, 1153, 1122, 1095, 978, 910, 736, 696; 1H NMR (400 MHz, CDCl3) 7.42–7.28 (5H, multiplet, H-3′–H-7′), 5.12 (2H, singlet, H-1′), 2.36 (1H, doublet of doublets, J = 14.7, 6.1 Hz, Ha-2), 2.16 (1H, doublet of doublets, J = 14.7, 8.2 Hz, Hb-2), 1.91 (1H, doublet of doublets of quartets of doublets of doublets, J = 8.2, 7.4, 6.7, 6.1, 5.7 Hz, H-3), 1.36 (1H, doublet of quartets of doublets, J = 13.2, 7.4, 5.7 Hz, Ha-4), 1.23 (1H, pseudo doublet of quintets, J = 13.2, 7.4 Hz, Hb-4), 0.93 (3H, doublet, J = 6.7 Hz, H-6), 0.88 (3H, triplet, J = 7.4 Hz, H-5); 13C NMR (101 MHz, CDCl3) 173.22 (C-1), 136.14 (C-2′), 128.52 (C-4′ and C-6′), 128.17 (C-3′ and C-7′), 128.14 (C-5′), 66.02 (C-1′), 41.47 (C-2), 31.95 (C-3), 29.33 (C-4), 19.27 (C-6), 11.27 (C-5); MS (EI), m/z (%) 206 (1), 108 (44), 107 (8), 97 (5), 92 (9), 91 (100), 90 (14), 79 (7), 73 (11), 71 (8), 43 (5).
Benzyl 4-methylpentanoate (2g), Yield: 80%; IR (cm−1) 2956, 2870, 1733, 1497, 1455, 1386, 1367, 1328, 1263, 1161, 1102, 1028, 970, 735, 696; 1H NMR (400 MHz, CDCl3) 7.40–7.29 (5H, multiplet, H-3′–H-7′), 5.11 (2H, singlet, H-1′), 2.39–2.34 (2H, multiplet, H-2), 1.64–1.51 (3H, overlapping peaks, H-3 and H-4), 0.89 (6H, doublet, J = 6.3 Hz, H-5 and H-6); 13C NMR (101 MHz, CDCl3) 173.88 (C-1), 136.10 (C-2′), 136.28 (C-2′), 128.53 (C-4′ and C-6′), 128.16 (C-3′, C-5′, and C-7′), 66.10 (C-1′), 33.72 (C-3), 32.40 (C-2), 27.67 (C-4), 22.22 (C-5 and C-6); MS (EI), m/z (%) 206 (1), 115 (23), 108 (44), 97 (15), 92 (17), 91 (100), 90 (11), 81 (11), 65 (12), 43 (13), 41 (9).
Benzyl hexanoate (2h),Yield: 78%; IR (cm−1) 2956, 2931, 2871, 1734, 1498, 1456, 1379, 1352, 1213, 1160, 1097, 1029, 994, 905, 734, 696; 1H NMR (400 MHz, CDCl3) 7.39–7.30 (5H, multiplet, H-3′–H-7′), 5.11 (2H, singlet, H-1′), 2.35 (2H, triplet, J = 7.6 Hz, H-2), 1.65 (2H, quintet, J = 7.6 Hz, H-3), 1.37–1.24 (4H, overlapping peaks, H-4 and H-5), 0.89 (3H, triplet, J = 6.9 Hz, H-6); 13C NMR (101 MHz, CDCl3) 173.73 (C-1), 136.14 (C-2′), 128.55 (C-4′ and C-6′), 128.17 (C-3′, C-5′, and C-7′), 66.08 (C-1′), 34.32 (C-2), 31.31 (C-4), 24.66 (C-3), 22.33 (C-5), 13.93 (C-6); MS (EI), m/z (%) 206 (6), 108 (40), 99 (16), 97 (7), 92 (23), 91 (100), 65 (19), 43 (10), 41 (11).
Phenethyl 2-ethylbutanoate (3a), Yield: 45%; IR (cm−1) 2963, 2934, 2876, 1730, 1674, 1605, 1497, 1456, 1385, 1363, 1267, 1228, 1171, 1145, 1085, 1045, 988, 812, 746, 698; 1H NMR (400 MHz, CDCl3) 7.32–7.27 (2H, multiplet, H-4′ and H-8′), 7.25–7.19 (3H, multiplet, H-5′–H-7′), 4.31 (2H, triplet, J = 7.0 Hz, H-1′), 2.94 (2H, triplet, J = 7.0 Hz, H-2′), 2.1801* (1H, triplet of triplets, J = 8.9, 5.5 Hz, H-2), 1.5911* (1H, doublet of doublets of doublets, J = −13.5, 8.9, 7.4 Hz, H-3b), 1.5906* (1H, doublet of doublets of doublets, J = −13.5, 8.9, 7.4 Hz, H-5b), 1.4912* (1H, doublet of doublets of doublets, J = −13.5, 7.4, 5.5 Hz, H-3a), 1.4908* (1H, doublet of doublets of doublets, J = −13.5, 7.4, 5.5 Hz, H-5a), 0.8301* (6H, triplet, J = 7.4 Hz, H-4 and H-6); 13C NMR (101 MHz, CDCl3) 176.17 (C-1), 137.90 (C-3′), 128.89 (C-5′ and C-7′), 128.43 (C-4′ and C-8′), 126.48 (C-6′), 64.48 (C-1′), 48.93 (C-2), 35.23 (C-2′), 25.00 (C-3 and C-5), 11.77 (C-4 and C-6); MS (EI), m/z (%) 105 (18), 104 (100), 91 (6), 79 (4), 78 (3), 77 (6), 71 (11), 55 (3), 43 (11), 41 (4). * The values of chemical shift and coupling constants were determined by a simulation of the 1H NMR spectrum (manual iterative full spin analysis).
Phenethyl 2,2-dimethylbutanoate (3b), Yield: 42%; IR (cm−1) 2969, 2931, 2856, 1726, 1604, 1497, 1474, 1454, 1380, 1362, 1239, 1047, 1019, 990, 890, 747, 698; 1H NMR (400 MHz, CDCl3) 7.32–7.27 (2H, multiplet, H-4′ and H-8′), 7.25–7.19 (3H, multiplet, H-5′–H-7′), 4.31 (2H, triplet, J = 7.0 Hz, H-1′), 2.94 (2H, triplet, J = 7.0 Hz, H-2′), 1.59 (2H, quartet, J = 7.5 Hz, H-3), 1.18 (6H, singlet, H-5 and H-6), 0.82 (3H, triplet, J = 7.5 Hz, H-4); 13C NMR (101 MHz, CDCl3) 177.80 (C-1), 137.90 (C-3′), 128.89 (C-5′ and C-7′), 128.43 (C-4′ and C-8′), 126.48 (C-6′), 64.48 (C-1′), 42.71 (C-2), 35.23 (C-2′), 33.36 (C-3), 24.66 (C-5 and C-6), 9.23 (C-4); MS (EI), m/z (%) 105 (20), 104 (100), 91 (5), 79 (4), 78 (3), 77 (6), 71 (17), 65 (3), 43 (11), 41 (4).
Phenethyl 3,3-dimethylbutanoate (3c), Yield: 60%; IR (cm−1) 2969, 2931, 2856, 1726, 1604, 1497, 1474, 1454, 1380, 1362, 1239, 1047, 1019, 990, 890, 747, 698; 1H NMR (400 MHz, CDCl3) 7.32–7.27 (2H, multiplet, H-4′ and H-8′), 7.24–7.19 (3H, multiplet, H-5′–H-7′), 4.28 (2H, triplet, J = 7.1 Hz, H-1′), 2.94 (2H, triplet, J = 7.1 Hz, H-2′), 2.17 (2H, singlet, H-2), 0.98 (9H, singlet, H-4–H-6); 13C NMR (101 MHz, CDCl3) 172.30 (C-1), 137.87 (C-3′), 128.87 (C-5′ and C-7′), 128.44 (C-4′ and C-8′), 126.49 (C-6′), 64.48 (C-1′), 47.97 (C-2), 35.16 (C-2′), 30.67 (C-3), 29.60 (C-4–C-6); MS (EI), m/z (%) 105 (45), 104 (100), 99 (5), 91 (8), 79 (5), 78 (4), 77 (7), 65 (4), 57 (17), 41 (6).
Phenethyl 2,3-dimethylbutanoate (3d), Yield: 68%; IR (cm−1) 2971, 2931, 2855, 1731, 1605, 1497, 1470, 1454, 1389, 1344, 1255, 1187, 1151, 1075, 1031, 988, 747, 698; 1H NMR (400 MHz, CDCl3) 7.33–7.27 (2H, multiplet, H-4′ and H-8′), 7.25–7.19 (3H, multiplet, H-5′–H-7′), 4.35–4.24 (2H, multiplet, H-1′), 2.94 (2H, triplet, J = 7.0 Hz, H-2′), 2.20 (1H, pseudo quintet, J = 7.0 Hz, H-2), 1.87 (1H, pseudo octet, J = 7.0 Hz, H-3), 1.07 (3H, doublet, J = 7.0 Hz, H-6), 0.87 (3H, doublet, J = 7.0 Hz, H-4), 0.85 (3H, doublet, J = 7.0 Hz, H-5); 13C NMR (101 MHz, CDCl3) 176.34 (C-1), 137.91 (C-2′), 128.89 (C-4′ and C-6′), 128.43 (C-3′ and C-7′), 126.48 (C-5′), 64.59 (C-1′), 46.19 (C-2), 35.18 (C-2′), 30.93 (C-3), 20.67 (C-4), 19.13 (C-5), 13.69 (C-6); MS (EI), m/z (%) 105 (26), 104 (100), 99 (8), 91 (7), 79 (6), 78 (6), 77 (8), 65 (5), 57 (12), 41 (5).
Phenethyl 2-methylpentanoate (3e), Yield: 62%; IR (cm−1) 2958, 2934, 2873, 1731, 1604, 1497, 1455, 1382, 1349, 1273, 1245, 1173, 1146, 1084, 1055, 1031, 746, 698; 1H NMR (400 MHz, CDCl3) 7.32–7.27 (2H, multiplet, H-4′ and H-8′), 7.25–7.19 (3H, multiplet, H-5′–H-7′), 4.29 (2H, multiplet, H-1′), 2.94 (2H, triplet, J = 7.0 Hz, H-2′), 2.50 (1H, pseudo sextet, J = 7.0 Hz, H-2), 1.65–1.54 (1H, multiplet, Ha-3), 1.41–1.30 (1H, multiplet, Hb-3), 1.29–1.19 (2H, multiplet, H-4), 1.10 (3H, doublet, J = 7.0 Hz, H-6), 0.87 (3H, triplet, J = 7.2 Hz, H-5); 13C NMR (101 MHz, CDCl3) 176.83 (C-1), 137.91 (C-3′), 128.91 (C-5′ and C-7′), 128.43 (C-4′ and C-8′), 126.48 (C-6′), 64.59 (C-1′), 39.32 (C-2), 35.92 (C-3), 35.17 (C-2′), 20.35 (C-4), 17.02 (C-6), 13.95 (C-5); MS (EI), m/z (%) 105 (18), 104 (100), 99 (4), 91 (4), 79 (6), 78 (4), 77 (5), 43 (9), 41 (12).
Phenethyl 3-methylpentanoate (3f), Yield: 75%; IR (cm−1) 2960, 2931, 2875, 1732, 1605, 1497, 1455, 1381, 1359, 1286, 1242, 1176, 1154, 1124, 1096, 1053, 1031, 1000, 748, 698; 1H NMR (400 MHz, CDCl3) 7.32–7.27 (2H, multiplet, H-4′ and H-8′), 7.24–7.19 (3H, multiplet, H-5′–H-7′), 4.29 (2H, triplet, J = 7.1 Hz, H-1′), 2.93 (2H, triplet, J = 7.1 Hz, H-2′), 2.28 (1H, doublet of doublets, J = 14.7, 6.1 Hz, Ha-2), 2.08 (1H, doublet of doublets, J = 14.7, 8.1 Hz, Hb-2), 1.85 (1H, doublet of doublets of quartets of doublets of doublets, J = 8.1, 7.4, 6.7, 6.1, 5.7 Hz, H-3), 1.32 (1H, doublet of quartets of doublets, J = 13.2, 7.4, 5.7 Hz, Ha-4), 1.19 (1H, pseudo doublet of quintets, J = 13.2, 7.4 Hz, Hb-4), 0.89 (3H, doublet, J = 6.7 Hz, H-6), 0.86 (3H, triplet, J = 7.4 Hz, H-5); 13C NMR (101 MHz, CDCl3) 173.32 (C-1), 137.90 (C-3′), 128.90 (C-5′ and C-7′), 128.47 (C-4′ and C-8′), 126.52 (C-6′), 64.63 (C-1′), 41.51 (C-2), 35.18 (C-2′), 31.91 (C-3), 29.30 (C-4), 19.25 (C-6), 11.28 (C-5); MS (EI), m/z (%) 105 (26), 104 (100), 99 (5), 91 (7), 79 (4), 78 (3), 77 (6), 43 (7), 41 (5).
Phenethyl 4-methylpentanoate (3g), Yield: 80%; IR (cm−1) 2956, 2870, 1732, 1605, 1497, 1468, 1454, 1386, 1367, 1329, 1245, 1164, 1103, 1055, 1031, 999, 748, 698; 1H NMR (400 MHz, CDCl3) 7.33–7.20 (5H, multiplet, H-4′–H-8′), 4.29 (2H, triplet, J = 7.1, Hz H-1′), 2.94 (2H, triplet, J = 7.1 Hz, H-2′), 2.32–2.26 (2H, multiplet, H-2), 1.58–1.44 (3H, overlapping peaks, H-3 and H-4), 0.88 (6H, doublet, J = 6.3 Hz, H-5 and H-6); 13C NMR (101 MHz, CDCl3) 174.01 (C-1), 137.89 (C-3′), 128.91 (C-5′ and C-7′), 128.47 (C-4′ and C-8′), 126.53 (C-6′), 64.73 (C-1′), 35.15 (C-2′), 33.75 (C-3), 32.39 (C-2), 27.63 (C-4), 22.22 (C-5 and C-6); MS (EI), m/z (%) 105 (27), 104 (100), 99 (3), 91 (7), 79 (5), 78 (4), 77 (6), 43 (10), 41 (5).
Phenethyl hexanoate (3h), Yield: 82%; IR (cm−1) 2955, 2930, 2860, 1732, 1604, 1497, 1454, 1381, 1353, 1242, 1165, 1098, 1031, 1001, 748, 698; 1H NMR (400 MHz, CDCl3) 7.33–7.28 (2H, multiplet, H-4′ and H-8′), 7.25–7.20 (3H, multiplet, H-5′–H-7′), 4.29 (2H, triplet, J = 7.1 Hz, H-1′), 2.94 (2H, triplet, J = 7.1 Hz, H-2′), 2.28 (2H, triplet, J = 7.5 Hz, H-2), 1.60 (2H, quintet, J = 7.5 Hz, H-3), 1.36–1.18 (4H, overlapping peaks, H-4 and H-5), 0.88 (3H, triplet, J = 7.0 Hz, H-6); 13C NMR (101 MHz, CDCl3) 173.82 (C-1), 137.88 (C-3′), 128.89 (C-5′ and C-7′), 128.46 (C-4′ and C-8′), 126.51 (C-6′), 64.69 (C-1′), 35.14 (C-2′), 34.30 (C-2), 31.27 (C-4), 24.63 (C-3), 22.32 (C-5), 13.91 (C-6); MS (EI), m/z (%) 105 (21), 104 (100), 99 (5), 91 (7), 79 (4), 78 (4), 77 (6), 43 (10), 41 (4).
3-Phenylpropyl 2-ethylbutanoate (4a), Yield: 52%; IR (cm−1) 2963, 2933, 2876, 1730, 1604, 1497, 1455, 1383, 1367, 1324, 1267, 1229, 1173, 1146, 1086, 1015, 945, 910, 743, 698; 1H NMR (400 MHz, CDCl3) 7.31–7.26 (2H, multiplet, H-6′ and H-8′), 7.22–7.16 (3H, multiplet, H-5′, H-7′, and H-9′), 4.11 (2H, triplet, J = 6.5 Hz, H-1′), 2.69 (2H, pseudo triplet, J = 7.5 Hz, H-3′), 2.2186* (1H, triplet of triplets, J = 8.9, 5.5 Hz, H-2), 2.00–1.92 (2H, multiplet, H-2′), 1.5912* (1H, doublet of doublets of doublets, J = −13.5, 8.9, 7.4 Hz, H-3b), 1.5906* (1H, doublet of doublets of doublets, J = −13.5, 8.9, 7.4 Hz, H-5b), 1.4914* (1H, doublet of doublets of doublets, J = −13.5, 7.4, 5.5 Hz, H-3a), 1.4907* (1H, doublet of doublets of doublets, J = −13.5, 7.4, 5.5 Hz, H-5a), 0.9101* (6H, triplet, J = 7.4 Hz, H-4 and H-6); 13C NMR (101 MHz, CDCl3) 176.29 (C-1), 141.24 (C-4′), 128.43 (C-6′ and C-8′), 128.40 (C-5′ and C-9′), 125.99 (C-7′), 63.32 (C-1′), 49.03 (C-2), 32.21 (C-3′), 30.42 (C-2′), 25.10 (C-3 and C-5), 11.89 (C-4 and C-6); MS (EI), m/z (%) 119 (10), 118 (100), 117 (78), 115 (3), 91 (33), 77 (4), 65 (6), 43 (12), 41 (6). * The values of chemical shift and coupling constants were determined by a simulation of the 1H NMR spectrum (manual iterative full spin analysis).
3-Phenylpropyl 2,2-dimethylbutanoate (4b), Yield: 48%; IR (cm−1) 2969, 2932, 2879, 1726, 1604, 1497, 1473, 1455, 1389, 1365, 1314, 1240, 1148, 1053, 1019, 989, 745, 698; 1H NMR (400 MHz, CDCl3) 7.31–7.26 (2H, multiplet, H-6′ and H-8′), 7.22–7.16 (3H, multiplet, H-5′, H-7′, and H-9′), 4.08 (2H, triplet, J = 6.4 Hz, H-1′), 2.69 (2H, doublet of doublets, J = 8.6, 6.8 Hz, H-3′), 2.00–1.91 (2H, multiplet, H-2′), 1.58 (2H, quartet, J = 7.5 Hz, H-3), 1.17 (6H, singlet, H-5 and H-6), 0.85 (3H, triplet, J = 7.5 Hz, H-4); 13C NMR (101 MHz, CDCl3) 177.99 (C-1), 141.26 (C-4′), 128.43 (C-6′ and C-8′), 128.40 (C-5′ and C-9′), 125.99 (C-7′), 63.45 (C-1′), 42.69 (C-2), 33.38 (C-3), 32.20 (C-3′), 30.37 (C-2′), 24.70 (C-5 and C-6), 9.32 (C-4); MS (EI), m/z (%) 119 (10), 118 (100), 117 (68), 92 (3), 91 (32), 77 (4), 65 (6), 43 (14), 41 (6).
3-Phenylpropyl 3,3-dimethylbutanoate (4c), Yield: 65%; IR (cm−1) 2955, 2868, 1730, 1604, 1497, 1473, 1465, 1454, 1366, 1321, 1226, 1197, 1128, 1046, 1019, 966, 912, 746, 698; 1H NMR (400 MHz, CDCl3) 7.31–7.26 (2H, multiplet, H-6′ and H-8′), 7.22–7.16 (3H, multiplet, H-5′, H-7′, and H-9′), 4.08 (2H, triplet, J = 6.6 Hz, H-1′), 2.69 (2H, pseudo triplet, J = 7.5 Hz, H-3′), 2.21 (2H, singlet, H-2), 2.00–1.91 (2H, multiplet, H-2′), 1.04 (9H, singlet, H-4–H-6); 13C NMR (101 MHz, CDCl3) 172.42 (C-1), 141.24 (C-4′), 128.42 (C-6′ and C-8′), 128.38 (C-5′ and C-9′), 125.98 (C-7′), 63.34 (C-1′), 48.04 (C-2), 32.28 (C-3′), 30.72 (C-3), 30.37 (C-2′), 29.47 (C-4–C-6); MS (EI), m/z (%) 119 (16), 118 (100), 117 (70), 115 (4), 92 (3), 91 (46), 77 (4), 65 (6), 41 (9).
3-Phenylpropyl 2,3-dimethylbutanoate (4d), Yield: 53%; IR (cm−1) 2930, 2855, 1731, 1604, 1497, 1453, 1389, 1345, 1298, 1257, 1188, 1152, 1122, 1076, 1045, 951, 890, 744, 698; 1H NMR (400 MHz, CDCl3) 7.31–7.26 (2H, multiplet, H-6′ and H-8′), 7.22–7.16 (3H, multiplet, H-5′, H-7′, and H-9′), 4.09 (2H, multiplet, H-1′), 2.69 (2H, doublet of doublets, J = 8.6, 6.8 Hz, H-3′), 2.24 (1H, quintet, J = 7.0 Hz, H-2), 2.00–1.86 (3H, overlapping peaks, H-3 and H-2′), 1.11 (3H, doublet, J = 7.0 Hz, H-6), 0.93 (6H, pseudo triplet, J = 6.7 Hz, H-4 and H-5); 13C NMR (101 MHz, CDCl3) 176.45 (C-1), 141.24 (C-4′), 128.42 (C-6′ and C-8′), 128.39 (C-5′ and C-9′), 125.98 (C-7′), 63.35 (C-1′), 46.26 (C-2), 32.22 (C-3′), 31.01 (C-3), 30.37 (C-2′), 20.75 (C-5), 19.21 (C-4), 13.79 (C-6); MS (EI), m/z (%) 119 (11), 118 (100), 117 (76), 92 (4), 91 (37), 77 (4), 71 (7), 65 (5), 43 (11), 41 (7).
3-Phenylpropyl 2-methylpentanoate (4e), Yield: 65%; IR (cm−1) 2957, 2934, 2873, 1731, 1604, 1497, 1454, 1379, 1273, 1240, 1174, 1147, 1085, 1054, 1029, 912, 743, 698; 1H NMR (400 MHz, CDCl3) 7.31–7.26 (2H, multiplet, H-6′ and H-8′), 7.24–7.14 (3H, multiplet, H-5′, H-7′, and H-9′), 4.12 (2H, multiplet, H-1′), 2.69 (2H, doublet of doublets, J = 8.7, 6.8 Hz, H-3′), 2.46 (1H, pseudo sextet, J = 7.0 Hz, H-2), 2.00–1.92 (2H, multiplet, H-2′), 1.71–1.59 (1H, multiplet, Ha-3), 1.45–1.27 (3H, overlapping peaks, Hb-3 and H-4), 1.15 (3H, doublet, J = 7.0 Hz, H-6), 0.92 (3H, triplet, J = 7.2 Hz, H-5); 13C NMR (101 MHz, CDCl3) 176.94 (C-1), 141.25 (C-4′), 128.43 (C-6′ and C-8′), 128.40 (C-5′ and C-9′), 125.99 (C-7′), 63.43 (C-1′), 39.38 (C-2), 35.99 (C-3), 32.18 (C-3′), 30.34 (C-2′), 20.44 (C-4), 17.11 (C-6), 13.98 (C-5); MS (EI), m/z (%) 119 (10), 118 (100), 117 (78), 92 (4), 91 (35), 77 (4), 71 (7), 65 (6), 43 (11), 41 (7).
3-Phenylpropyl 3-methylpentanoate (4f), Yield: 82%; IR (cm−1) 2959, 2875, 1732, 1496, 1454, 1380, 1363, 1243, 1177, 1125, 1095, 1019, 912, 745, 698; 1H NMR (400 MHz, CDCl3) 7.31–7.26 (2H, multiplet, H-6′ and H-8′), 7.22–7.16 (3H, multiplet, H-5′, H-7′, and H-9′), 4.09 (2H, triplet, J = 6.5 Hz, H-1′), 2.69 (2H, doublet of doublets, J = 8.7, 6.8 Hz, H-3′), 2.31 (1H, doublet of doublets, J = 14.6, 6.1 Hz, Ha-2), 2.11 (1H, doublet of doublets, J = 14.6, 8.1 Hz, Hb-2), 2.00 –1.83 (3H, overlapping peaks, H-3 and H-2′), 1.38 (1H, doublet of quartets of doublets, J = 13.2, 7.4, 5.7 Hz, Ha-4), 1.19 (1H, pseudo doublet of quintets, J = 13.2, 7.4 Hz, Hb-4), 0.94 (3H, doublet, J = 6.7 Hz, H-6), 0.90 (3H, triplet, J = 7.4 Hz, H-5); 13C NMR (101 MHz, CDCl3) 173.43 (C-1), 141.23 (C-4′), 128.42 (C-6′ and C-8′), 128.39 (C-5′ and C-9′), 125.99 (C-7′), 63.49 (C-1′), 41.53 (C-2), 32.22 (C-3′), 31.97 (C-3), 30.31 (C-2′), 29.34 (C-4), 19.30 (C-6), 11.30 (C-5); MS (EI), m/z (%) 119 (11), 118 (100), 117 (77), 92 (4), 91 (40), 77 (4), 71 (4), 65 (6), 43 (7), 41 (8).
3-Phenylpropyl 4-methylpentanoate (4g), Yield: 90%; IR (cm−1) 2955, 2869, 1732, 1603, 1497, 1454, 1386, 1367, 1329, 1247, 1166, 1104, 1028, 745, 698; 1H NMR (400 MHz, CDCl3) 7.32–7.26 (2H, multiplet, H-6′ and H-8′), 7.22–7.16 (3H, multiplet, H-5′, H-7′, and H-9′), 4.09 (2H, triplet, J = 7.1 Hz, H-1′), 2.69 (2H, doublet of doublets, J = 8.6, 6.8 Hz, H-3′), 2.34–2.27 (2H, multiplet, H-2), 2.00–1.92 (2H, multiplet, H-2′), 1.62–1.49 (3H, overlapping peaks, H-3 and H-4), 0.91 (6H, doublet, J = 6.3 Hz, H-5 and H-6); 13C NMR (101 MHz, CDCl3) 174.10 (C-1), 141.24 (C-4′), 128.42 (C-6′ and C-8′), 128.38 (C-5′ and C-9′), 125.98 (C-7′), 63.60 (C-1′), 33.82 (C-3), 32.42 (C-2), 32.41 (C-3′), 30.26 (C-2′), 27.69 (C-4), 22.24 (C-5 and C-6); MS (EI), m/z (%) 119 (12), 118 (100), 117 (78), 92 (4), 91 (40), 81 (4), 77 (4), 65 (5), 43 (10), 41 (7).
3-Phenylpropyl hexanoate (4h), Yield: 85%; IR (cm−1) 2955, 2930, 2860, 1733, 1604, 1496, 1454, 1389, 1360, 1243, 1167, 1098, 1020, 910, 744, 698; 1H NMR (400 MHz, CDCl3) 7.31–7.26 (2H, multiplet, H-6′ and H-8′), 7.22–7.16 (3H, multiplet, H-5′, H-7′, and H-9′), 4.09 (2H, triplet, J = 6.5 Hz, H-1′), 2.69 (2H, doublet of doublets, J = 8.6, 6.8 Hz, H-3′), 2.30 (2H, triplet, J = 7.5 Hz, H-2), 2.00–1.91 (2H, multiplet, H-2′), 1.63 (2H, quintet, J = 7.5 Hz, H-3), 1.39–1.23 (4H, overlapping peaks, H-4 and H-5), 0.90 (3H, triplet, J = 7.0 Hz, H-6); 13C NMR (101 MHz, CDCl3) 173.92 (C-1), 141.24 (C-4′), 128.42 (C-6′ and C-8′), 128.39 (C-5′ and C-9′), 125.98 (C-7′), 63.56 (C-1′), 34.33 (C-2), 32.20 (C-3′), 31.34 (C-4), 30.27 (C-2′), 24.71 (C-3), 22.34 (C-5), 13.93 (C-6); MS (EI), m/z (%) 119 (10), 118 (100), 117 (80), 115 (4), 92 (4), 91 (37), 77 (4), 65 (5), 43 (8), 41 (7).
4-Phenylbutyl 2-ethylbutanoate (5a), Yield: 55%; IR (cm−1) 2955, 2867, 1733, 1604, 1496, 1454, 1381, 1360, 1167, 1021, 991, 746, 698; 1H NMR (400 MHz, CDCl3) 7.31–7.26 (2H, multiplet, H-7′ and H-9′), 7.21–7.15 (3H, multiplet, H-6′, H-8′, and H-10′), 4.11 (2H, multiplet, H-1′), 2.65 (2H, multiplet, H-4′), 2.1901* (1H, triplet of triplets, J = 8.9, 5.5 Hz, H-2), 1.75–1.66 (4H, overlapping peaks, H-2′ and H-3′), 1.5913* (1H, doublet of doublets of doublets, J = −13.5, 8.9, 7.4 Hz, H-3b), 1.5905* (1H, doublet of doublets of doublets, J = −13.5, 8.9, 7.4 Hz, H-5b), 1.4919* (1H, doublet of doublets of doublets, J = −13.5, 7.4, 5.5 Hz, H-3a), 1.4912* (1H, doublet of doublets of doublets, J = −13.5, 7.4, 5.5 Hz, H-5a), 0.8802* (6H, triplet, J = 7.4 Hz, H-4 and H-6); 13C NMR (101 MHz, CDCl3) 176.36 (C-1), 142.07 (C-5′), 128.37 (C-7′ and C-9′), 128.33 (C-6′ and C-10′), 125.81 (C-8′), 63.86 (C-1′), 49.02 (C-2), 35.45 (C-4′), 28.33 and 27.78 (C-2′ and C-3′), 25.08 (C-3 and C-5), 11.86 (C-4 and C-6); MS (EI), m/z (%) 248 (1), 132 (12), 131 (5), 117 (10), 105 (11), 104 (100), 91 (39), 71 (30), 65 (5), 43 (11), 41 (5). * The values of chemical shift and coupling constants were determined by a simulation of the 1H NMR spectrum (manual iterative full spin analysis).
4-Phenylbutyl 2,2-dimethylbutanoate (5b), Yield: 51%; IR (cm−1) 2969, 2935, 2862, 1726, 1604, 1496, 1473, 1460, 1454, 1388, 1365, 1314, 1240, 1149, 1063, 1019, 990, 746, 698; 1H NMR (400 MHz, CDCl3) 7.31–7.26 (2H, multiplet, H-7′ and H-9′), 7.21–7.15 (3H, multiplet, H-6′, H-8′, and H-10′), 4.07 (2H, multiplet, H-1′), 2.64 (2H, multiplet, H-4′), 1.74–1.63 (4H, overlapping peaks, H-2′ and H-3′), 1.56 (2H, quartet, J = 7.5 Hz, H-3), 1.15 (6H, singlet, H-5 and H-6), 0.83 (3H, triplet, J = 7.5 Hz, H-4); 13C NMR (101 MHz, CDCl3) 178.10 (C-1), 142.09 (C-5′), 128.36 (C-7′ and C-9′), 128.33 (C-6′ and C-10′), 125.80 (C-8′), 64.06 (C-1′), 42.65 (C-2), 35.44 (C-4′), 33.37 (C-3), 28.27 and 27.77 (C-2′ and C-3′), 24.68 (C-5 and C-6), 9.29 (C-4); MS (EI), m/z (%) 248 (1), 132 (18), 131 (4), 117 (12), 105 (10), 104 (100), 91 (49), 71 (31), 65 (6), 43 (16), 41 (6).
4-Phenylbutyl 3,3-dimethylbutanoate (5c), Yield: 81%; IR (cm−1) 2953, 2866, 1730, 1604, 1496, 1473, 1465, 1454, 1322, 1226, 1128, 1046, 746, 698; 1H NMR (400 MHz, CDCl3) 7.31–7.25 (2H, multiplet, H-7′ and H-9′), 7.21–7.15 (3H, multiplet, H-6′, H-8′, and H-10′), 4.08 (2H, multiplet, H-1′), 2.64 (2H, pseudo triplet, J = 7.2 Hz, H-4′), 2.19 (2H, singlet, H-2), 1.74–1.62 (4H, overlapping peaks, H-2′ and H-3′), 1.02 (9H, singlet, H-4–H-6); 13C NMR (101 MHz, CDCl3) 172.46 (C-1), 142.03 (C-5′), 128.36 (C-7′ and C-9′), 128.32 (C-6′ and C-10′), 125.80 (C-8′), 63.83 (C-1′), 48.03 (C-2), 35.43 (C-4′), 30.70 (C-3), 29.65 (C-4–C-6), 28.27 and 27.83 (C-2′ and C-3′); MS (EI), m/z (%) 248 (1), 133 (10), 132 (21), 117 (12), 105 (10), 104 (100), 99 (14), 91 (68), 65 (7), 41 (10).
4-Phenylbutyl 2,3-dimethylbutanoate (5d), Yield: 64%; IR (cm−1) 2930, 2854, 1732, 1604, 1496, 1450, 1389, 1359, 1346, 1298, 1188, 1151, 1075, 1045, 891, 746, 698; 1H NMR (400 MHz, CDCl3) 7.31–7.26 (2H, multiplet, H-7′ and H-9′), 7.21–7.15 (3H, multiplet, H-6′, H-8′, and H-10′), 4.14–4.03 (2H, multiplet, H-1′), 2.64 (2H, multiplet, H-4′), 2.22 (1H, quintet, J = 7.0 Hz, H-2), 1.90 (1H, pseudo octet, J = 7.0 Hz, H-3), 1.75–1.60 (4H, overlapping peaks, H-2′, and H-3′), 1.09 (3H, doublet, J = 7.0 Hz, H-6), 0.91 (3H, doublet, J = 7.0 Hz, H-4), 0.90 (3H, doublet, J = 7.0 Hz, H-5); 13C NMR (101 MHz, CDCl3) 176.50 (C-1), 142.07 (C-5′), 128.37 (C-7′ and C-9′), 128.33 (C-6′ and C-10′), 125.81 (C-8′), 63.89 (C-1′), 46.26 (C-2), 35.46 (C-4′), 31.01 (C-3), 28.30 and 27.78 (C-2′ and C-3′), 20.73 (C-5), 19.20 (C-4), 13.77 (C-6); MS (EI), m/z (%) 248 (1), 132 (19), 117 (13), 105 (10), 104 (100), 99 (8), 91 (54), 71 (18), 65 (6), 43 (13), 41 (6).
4-Phenylbutyl 2-methylpentanoate (5e), Yield: 63%; IR (cm−1) 2956, 2934, 2872, 1731, 1604, 1496, 1454, 1378, 1352, 1240, 1175, 1147, 1085, 1031, 745, 698; 1H NMR (400 MHz, CDCl3) 7.31–7.26 (2H, multiplet, H-7′ and H-9′), 7.21–7.15 (3H, multiplet, H-6′, H-8′, and H-10′), 4.08 (2H, multiplet, H-1′), 2.65 (2H, multiplet, H-4′), 2.43 (1H, pseudo sextet, J = 7.0 Hz, H-2), 1.75–1.60 (5H, overlapping peaks, Ha-3, H-2′, and H-3′), 1.44–1.25 (3H, overlapping peaks, Hb-3 and H-4), 1.13 (3H, doublet, J = 7.0 Hz, H-6), 0.90 (3H, triplet, J = 7.2 Hz, H-5); 13C NMR (101 MHz, CDCl3) 177.00 (C-1), 142.08 (C-5′), 128.37 (C-7′ and C-9′), 128.33 (C-6′ and C-10′), 125.81 (C-8′), 63.98 (C-1′), 39.37 (C-2), 35.98 (C-3), 35.45 (C-4′), 28.28 and 27.75 (C-2′ and C-3′), 20.42 (C-4), 17.09 (C-6), 13.97 (C-5); MS (EI), m/z (%) 248 (1), 132 (17), 117 (12), 105 (10), 104 (100), 99 (6), 91 (49), 71 (16), 65 (6), 43 (12), 41 (6).
4-Phenylbutyl 3-methylpentanoate (5f), Yield: 92%; IR (cm−1) 2959, 2874, 1731, 1604, 1496, 1454, 1380, 1286, 1243, 1178, 1125, 1095, 1030, 746, 698; 1H NMR (400 MHz, CDCl3) 7.31–7.25 (2H, multiplet, H-7′ and H-9′), 7.21–7.15 (3H, multiplet, H-6′, H-8′, and H-10′), 4.08 (2H, multiplet, H-1′), 2.64 (2H, multiplet, H-4′), 2.30 (1H, doublet of doublets, J = 14.6, 6.1 Hz, Ha-2), 2.11 (1H, doublet of doublets, J = 14.6, 8.1 Hz, Hb-2), 1.87 (1H, doublet of doublets of quartets of doublets of doublets, J = 8.1, 7.4, 6.7, 6.1, 5.7 Hz, H-3), 1.74–1.62 (4H, overlapping peaks, H-2′, and H-3′), 1.36 (1H, doublet of quartets of doublets, J = 13.2, 7.4, 5.7 Hz, Ha-4), 1.22 (1H, pseudo doublet of quintets, J = 13.2, 7.4 Hz, Hb-4), 0.92 (3H, doublet, J = 6.7 Hz, H-6), 0.90 (3H, triplet, J = 7.4 Hz, H-5); 13C NMR (101 MHz, CDCl3) 173.47 (C-1), 142.05 (C-5′), 128.37 (C-7′ and C-9′), 128.33 (C-6′ and C-10′), 125.81 (C-8′), 64.01 (C-1′), 41.54 (C-2), 35.45 (C-4′), 31.95 (C-3), 29.34 (C-4), 28.27 and 27.77 (C-2′ and C-3′), 19.28 (C-6), 11.28 (C-5); MS (EI), m/z (%) 248 (1), 132 (19), 117 (12), 105 (10), 104 (100), 99 (12), 91 (54), 71 (8), 43 (7), 41 (6).
4-Phenylbutyl 4-methylpentanoate (5g), Yield: 95%; IR (cm−1) 2954, 2869, 1732, 1604, 1496, 1453, 1386, 1367, 1329, 1265, 1166, 1103, 1065, 1030, 746, 698; 1H NMR (400 MHz, CDCl3) 7.31–7.25 (2H, multiplet, H-7′ and H-9′), 7.21–7.16 (3H, multiplet, H-6′, H-8′, and H-10′), 4.08 (2H, multiplet, H-1′), 2.64 (2H, multiplet, H-4′), 2.32–2.27 (2H, multiplet, H-2), 1.74–1.61 (4H, overlapping peaks, H-2′, and H-3′), 1.60–1.49 (3H, overlapping peaks, H-3 and H-4), 0.89 (6H, doublet, J = 6.3 Hz, H-5 and H-6); 13C NMR (101 MHz, CDCl3) 174.14 (C-1), 142.04 (C-5′), 128.37 (C-7′ and C-9′), 128.32 (C-6′ and C-10′), 125.81 (C-8′), 64.11 (C-1′), 35.44 (C-4′), 33.80 (C-3), 32.43 (C-2), 28.23 and 27.74 (C-2′ and C-3′), 27.68 (C-4), 22.23 (C-5 and C-6); MS (EI), m/z (%) 248 (1), 132 (18), 131 (8), 117 (12), 105 (10), 104 (100), 99 (7), 91 (53), 81 (8), 43 (10), 41 (8).
4-Phenylbutyl hexanoate (5h), Yield: 90%; IR (cm−1) 2931, 2860, 1732, 1604, 1496, 1454, 1244, 1167, 1097, 1065, 1030, 746, 698; 1H NMR (400 MHz, CDCl3) 7.31–7.25 (2H, multiplet, H-7′ and H-9′), 7.23–7.14 (3H, multiplet, H-6′, H-8′, and H-10′), 4.09 (2H, pseudo triplet, J = 6.5 Hz, H-1′), 2.64 (2H, pseudo triplet, J = 7.2 Hz, H-4′), 2.28 (2H, triplet, J = 7.5 Hz, H-2), 1.75–1.55 (6H, overlapping peaks, H-2′, H-3′, and H-3), 1.39–1.24 (4H, overlapping peaks, H-4 and H-5), 0.89 (3H, triplet, J = 7.0 Hz, H-6); 13C NMR (101 MHz, CDCl3) 173.97 (C-1), 142.05 (C-5′), 128.37 (C-7′ and C-9′), 128.33 (C-6′ and C-10′), 125.81 (C-8′), 64.08 (C-1′), 35.45 (C-4′), 34.34 (C-2), 31.33 (C-4), 28.25 and 27.76 (C-2′ and C-3′), 24.70 (C-3), 22.33 (C-5), 13.92 (C-6); MS (EI), m/z (%) 248 (1), 132 (17), 131 (9), 117 (12), 105 (10), 104 (100), 99 (9), 91 (48), 65 (6), 43 (10), 41 (6).
5-Phenylpentyl 2-ethylbutanoate (6a), Yield: 58%; IR (cm−1) 2931, 2855, 1730, 1604, 1495, 1454, 1240, 1155, 1020, 747, 698; 1H NMR (400 MHz, CDCl3) 7.30–7.24 (2H, multiplet, H-8′ and H-10′), 7.22–7.13 (3H, multiplet, H-7′, H-9′, and H-11′), 4.04 (2H, triplet, J = 6.7 Hz, H-1′), 2.62 (2H, pseudo triplet, J = 7.6 Hz, H-5′), 2.4571* (1H, triplet of triplets, J = 8.9, 5.5 Hz, H-2), 1.7812* (1H, doublet of doublets of doublets, J = −13.5, 8.9, 7.4 Hz, H-3b), 1.7803* (1H, doublet of doublets of doublets, J = −13.5, 8.9, 7.4 Hz, H-5b), 1.70–1.59 (6H, overlapping peaks, H-3a, H-5a, H-2′ and H-4′), 1.44–1.35 (2H, multiplet, H-3′), 1.0242* (6H, triplet, J = 7.4 Hz, H-4 and H-6); 13C NMR (101 MHz, CDCl3) 176.36 (C-1), 142.40 (C-6′), 128.37 (C-8′ and C-10′), 128.27 (C-7′ and C-11′), 125.68 (C-9′), 64.17 (C-1′), 49.02 (C-2), 35.78 (C-5′), 31.08 (C-4′), 28.54 (C-2′), 25.63 (C-3′), 25.08 (C-3 and C-5), 11.86 (C-4 and C-6); MS (EI), m/z (%) 146 (92), 131 (13), 118 (19), 117 (79), 105 (20), 104 (94), 92 (17), 91 (100), 71 (29), 43 (26). *The values of chemical shift and coupling constants were determined by a simulation of the 1H NMR spectrum (manual iterative full spin analysis).
5-Phenylpentyl 2,2-dimethylbutanoate (6b), Yield: 55%; IR (cm−1) 2970, 2929, 2856, 1728, 1451, 1388, 1360, 1346, 1311, 1240, 1150, 1018, 989, 748, 698; 1H NMR (400 MHz, CDCl3) 7.31–7.26 (2H, multiplet, H-8′ and H-10′), 7.22–7.15 (3H, multiplet, H-7′, H-9′, and H-11′), 4.05 (2H, triplet, J = 6.6 Hz, H-1′), 2.62 (2H, pseudo triplet, J =7.7 Hz, H-5′), 1.74–1.58 (4H, overlapping peaks, H-2′ and H-4′), 1.53 (2H, quartet, J = 7.5 Hz, H-3), 1.44–1.31 (2H, multiplet, H-3′), 1.13 (6H, singlet, H-5 and H-6), 0.83 (3H, triplet, J = 7.5 Hz, H-4); 13C NMR (101 MHz, CDCl3) 176.66 (C-1), 142.40 (C-6′), 128.37 (C-8′ and C-10′), 128.26 (C-7′ and C-11′), 125.68 (C-9′), 64.17 (C-1′), 42.18 (C-2), 35.78 (C-5′), 33.96 (C-3), 31.01 (C-4′), 28.52 (C-2′), 25.61 (C-3′), 24.98 (C-5 and C-6), 9.15 (C-4); MS (EI), m/z (%) 146 (93), 118 (18), 117 (76), 105 (19), 104 (94), 92 (16), 91 (100), 71 (47), 43 (34), 41 (13).
5-Phenylpentyl 3,3-dimethylbutanoate (6c), Yield: 84%; IR (cm−1) 2933, 2859, 1730, 1604, 1496, 1465, 1453, 1366, 1322, 1227, 1129, 1046, 1003, 746, 698; 1H NMR (400 MHz, CDCl3) 7.30–7.24 (2H, multiplet, H-8′ and H-10′), 7.22–7.13 (3H, multiplet, H-7′, H-9′, and H-11′), 4.04 (2H, triplet, J = 6.7 Hz, H-1′), 2.62 (2H, pseudo triplet, J =7.6 Hz, H-5′), 2.18 (2H, singlet, H-2), 1.70–1.59 (4H, overlapping peaks, H-2′ and H-4′), 1.44–1.35 (2H, multiplet, H-3′), 1.02 (9H, singlet, H-4–H-6); 13C NMR (101 MHz, CDCl3) 172.44 (C-1), 142.37 (C-6′), 128.36 (C-8′ and C-10′), 128.26 (C-7′ and C-11′), 125.68 (C-9′), 63.96 (C-1′), 48.05 (C-2), 35.78 (C-5′), 31.08 (C-4′), 30.68 (C-3), 29.64 (C-4–C-6), 28.54 (C-2′), 25.63 (C-3′); MS (EI), m/z (%) 146 (71), 118 (15), 117 (55), 105 (16), 104 (66), 99 (14), 92 (14), 91 (100), 57 (31), 41 (14).
5-Phenylpentyl 2,3-dimethylbutanoate (6d), Yield: 68%; IR (cm−1) 2929, 2855, 1731, 1604, 1496, 1451, 1389, 1359, 1345, 1298, 1258, 1189, 1152, 1075, 1045, 953, 746, 698; 1H NMR (400 MHz, CDCl3) 7.30–7.24 (2H, multiplet, H-8′ and H-10′), 7.20–7.14 (3H, multiplet, H-7′, H-9′, and H-11′), 4.12–3.99 (2H, multiplet, H-1′), 2.62 (2H, pseudo triplet, J =7.7 Hz, H-5′), 2.21 (1H, pseudo quintet, J = 7.0 Hz, H-2), 1.90 (1H, pseudo octet, J = 7.0 Hz, H-3), 1.70–1.60 (4H, overlapping peaks, H-2′ and H-4′), 1.44–1.35 (2H, multiplet, H-3′), 1.09 (3H, doublet, J = 7.0 Hz, H-6), 0.91 (3H, doublet, J = 7.0 Hz, H-5), 0.89 (3H, doublet, J = 7.0 Hz, H-4); 13C NMR (101 MHz, CDCl3) 176.50 (C-1), 142.39 (C-6′), 128.37 (C-8′ and C-10′), 128.27 (C-7′ and C-11′), 125.68 (C-9′), 64.02 (C-1′), 46.25 (C-2), 35.79 (C-5′), 31.03 (C-4′), 31.00 (C-3), 28.55 (C-2′), 25.59 (C-3′), 20.71 (C-5), 19.19 (C-4), 13.75 (C-6); MS (EI), m/z (%) 146 (86), 118 (17), 117 (69), 105 (19), 104 (80), 92 (16), 91 (100), 71 (28), 43 (23), 41 (12).
5-Phenylpentyl 2-methylpentanoate (6e), Yield: 62%; IR (cm−1) 2930, 2857, 1731, 1604, 1496, 1453, 1378, 1347, 1240, 1177, 1147, 1085, 1046, 955, 745, 698; 1H NMR (400 MHz, CDCl3) 7.31–7.24 (2H, multiplet, H-8′ and H-10′), 7.22–7.13 (3H, multiplet, H-7′, H-9′, and H-11′), 4.06 (2H, multiplet, H-1′), 2.62 (2H, pseudo triplet, J = 7.6 Hz, H-5′), 2.43 (1H, pseudo sextet, J = 7.0 Hz, H-2), 1.71–1.56 (5H, overlapping peaks, Ha-3, H-2′, and H-4′), 1.44–1.26 (5H, overlapping peaks, Hb-3, H-3′, and H-4), 1.12 (3H, doublet, J = 7.0 Hz, H-6), 0.90 (3H, triplet, J = 7.2 Hz, H-5); 13C NMR (101 MHz, CDCl3) 177.00 (C-1), 142.39 (C-6′), 128.37 (C-8′ and C-10′), 128.26 (C-7′ and C-11′), 125.68 (C-9′), 64.11 (C-1′), 39.36 (C-2), 35.97 (C-3), 35.79 (C-5′), 31.04 (C-4′), 28.53 (C-2′), 25.56 (C-3′), 20.41 (C-4), 17.08 (C-6), 13.97 (C-5); MS (EI), m/z (%) 146 (81), 118 (18), 117 (73), 105 (19), 104 (89), 92 (17), 91 (100), 71 (27), 43 (24), 41 (13).
5-Phenylpentyl 3-methylpentanoate (6f), Yield: 75%; IR (cm−1) 2960, 2930, 2857, 1732, 1604, 1496, 1454, 1380, 1360, 1242, 1179, 1124, 1097, 1040, 746, 698; 1H NMR (400 MHz, CDCl3) 7.30–7.24 (2H, multiplet, H-8′ and H-10′), 7.20–7.14 (3H, multiplet, H-7′, H-9′, and H-11′), 4.07 (2H, triplet, J = 6.7 Hz, H-1′), 2.64 (2H, pseudo triplet, J = 7.6 Hz, H-5′), 2.29 (1H, doublet of doublets, J = 14.6, 6.1 Hz, Ha-2), 2.09 (1H, doublet of doublets, J = 14.6, 8.1 Hz, Hb-2), 1.87 (1H, doublet of doublets of quartets of doublets of doublets, J = 8.1, 7.4, 6.7, 6.1, 5.7 Hz, H-3), 1.70–1.60 (4H, overlapping peaks, H-2′ and H-4′), 1.44–1.30 (3H, overlapping peaks, Ha-4 and H-3′), 1.22 (1H, pseudo doublet of quintets, J = 13.3, 7.4 Hz, Hb-4), 0.92 (3H, doublet, J = 6.7 Hz, H-6), 0.89 (3H, triplet, J = 7.4 Hz, H-5); 13C NMR (101 MHz, CDCl3) 173.47 (C-1), 142.40 (C-6′), 128.37 (C-8′ and C-10′), 128.27 (C-7′ and C-11′), 125.69 (C-9′), 64.14 (C-1′), 41.56 (C-2), 35.79 (C-5′), 31.95 (C-3), 31.05 (C-4′), 29.34 (C-4), 28.53 (C-2′), 25.59 (C-3′), 19.27 (C-6), 11.28 (C-5); MS (EI), m/z (%) 146 (75), 118 (17), 117 (65), 105 (18), 104 (78), 99 (15), 92 (16), 91 (100), 71 (15), 43 (14).
5-Phenylpentyl 4-methylpentanoate (6g), Yield: 80%; IR (cm−1) 2954, 2931, 2860, 1733, 1604, 1496, 1454, 1386, 1329, 1265, 1166, 1104, 1030, 745, 698; 1H NMR (400 MHz, CDCl3) 7.30–7.24 (2H, multiplet, H-8′ and H-10′), 7.20–7.15 (3H, multiplet, H-7′, H-9′, and H-11′), 4.05 (2H, triplet, J = 6.7 Hz, H-1′), 2.62 (2H, pseudo triplet, J =7.6 Hz, H-5′), 2.32–2.26 (2H, multiplet, H-2), 1.69–1.47 (7H, overlapping peaks, H-2′, H-4′, H-3 and H-4), 1.44–1.34 (2H, multiplet, H-3′), 0.89 (6H, doublet, J = 6.3 Hz, H-5 and H-6); 13C NMR (101 MHz, CDCl3) 174.14 (C-1), 142.39 (C-6′), 128.36 (C-8′ and C-10′), 128.27 (C-7′ and C-11′), 125.69 (C-9′), 64.25 (C-1′), 35.78 (C-5′), 33.80 (C-3), 32.44 (C-2), 31.06 (C-4′), 28.50 (C-2′), 27.68 (C-4), 25.57 (C-3′), 22.23 (C-5 and C-6); MS (EI), m/z (%) 146 (74), 118 (16), 117 (67), 105 (18), 104 (79), 92 (15), 91 (100), 81 (15), 43 (20), 41 (13).
5-Phenylpentyl hexanoate (6h), Yield: 82%; IR (cm−1) 2930, 2858, 1733, 1604, 1496, 1454, 1353, 1244, 1167, 1098, 1031, 745, 698; 1H NMR (400 MHz, CDCl3) 7.30–7.24 (2H, multiplet, H-8′ and H-10′), 7.20–7.14 (3H, multiplet, H-7′, H-9′, and H-11′), 4.05 (2H, triplet, J = 6.7 Hz, H-1′), 2.61 (2H, pseudo triplet, J = 7.6 Hz, H-5′), 2.28 (2H, triplet, J = 7.5 Hz, H-2), 1.70–1.57 (6H, overlapping peaks, H-2′, H-4′, and H-3), 1.44–1.26 (2H, multiplet, H-3′), 1.36–1.24 (4H, overlapping peaks, H-4 and H-5), 0.89 (3H, triplet, J = 6.9 Hz, H-6); 13C NMR (101 MHz, CDCl3) 173.95 (C-1), 142.40 (C-6′), 128.37 (C-8′ and C-10′), 128.27 (C-7′ and C-11′), 125.69 (C-8′), 64.21 (C-1′), 35.80 (C-5′), 34.35 (C-2), 31.33 (C-4), 31.06 (C-4′), 28.53 (C-2′), 25.59 (C-3′), 24.70 (C-3), 22.33 (C-5), 13.93 (C-6); MS (EI), m/z (%) 146 (79), 131 (12), 118 (18), 117 (73), 105 (19), 104 (87), 99 (14), 92 (17), 91 (100), 43 (18).