Evaluation of the Antibacterial Activity of Gentamicin in Combination with Essential Oils Isolated from Different Cultivars and Morphological Parts of Lavender (Lavandula angustifolia Mill.) against Selected Bacterial Strains
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
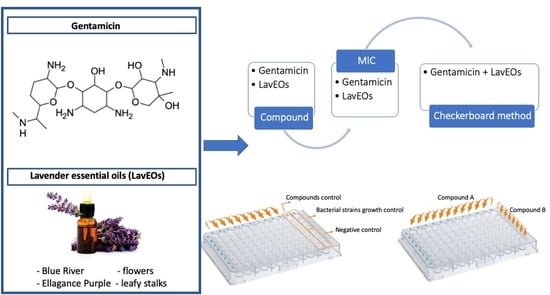
4.1. Isolation of Essential Oils
4.2. Microdilution Checkerboard Method
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Yap, P.S.; Yiap, B.C.; Ping, H.C.; Lim, S.H. Essential oils, a new horizon in combating bacterial antibiotic resistance. Open Microbiol. J. 2014, 8, 6–14. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.; Bano, N.; Ahmad, T.; Sharangi, A.B.; Upadhyay, T.K.; Alraey, Y.; Alabdallah, N.M.; Rauf, M.A.; Saeed, M. Synergistic Role of Plant Extracts and Essential Oils against Multidrug Resistance and Gram-Negative Bacterial Strains Producing Extended-Spectrum β-Lactamases. Antibiotics 2022, 11, 855. [Google Scholar] [CrossRef]
- Jia, J.; Feng, Z.; Xiaohua, M.; Zhiwei, W.C.; Yixue, X.L.; Yu, Z.C. Mechanisms of drug combinations: Interaction and network perspectives. Nat. Rev. Drug Discov. 2009, 8, 111–128. [Google Scholar] [CrossRef]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential Oils as Antimicrobial Agents-Myth or Real Alternative? Molecules 2019, 11, 2130. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, A.M.; El-Baky, R.M.A.; Ahmed, A.B.F.; Gad, G.F.M. Antibacterial Activity of Essential Oils and in Combination with Some Standard Antimicrobials against Different Pathogens Isolated from Some Clinical Specimens. Am. J. Microbiol. Res. 2016, 1, 16–25. [Google Scholar]
- Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Alqubaisy, M.; AlAli, M.; Molouki, A.; Ong-Abdullah, J.; Abushelaibi, A.; Lai, K.S.; Lim, S.E. An Overview of the Potential Therapeutic Applications of Essential Oils. Molecules 2021, 3, 628. [Google Scholar] [CrossRef]
- Adaszyńska-Skwirzyńska, M.; Szczerbińska, D.; Zych, S. Antibacterial activity of lavender essential oil and linalool combined with gentamicin on selected bacterial strains. Med. Wet. 2020, 176, 15–118. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Denys, P.; Kowalczyk, E. Antibacterial and immunostimulatory effect of essential oils. Int. Rev. Allergol. Clin. Immunol. 2011, 17, 40–44. [Google Scholar]
- Tardugno, R.; Serio, A.; Pellati, F.; D’Amato, S.; Chaves López, C.; Bellardi, M.G.; Di Vito, M.; Savini, V.; Paparella, A.; Benvenuti, S. Lavandula x intermedia and Lavandula angustifolia essential oils: Phytochemical composition and antimicrobial activity against foodborne pathogens. Nat. Prod. Res. 2019, 22, 3330–3335. [Google Scholar] [CrossRef]
- De Martino, L.; De Feo, V.; Nazzaro, F. Chemical composition and in vitro antimicrobial and mutagenic activities of seven Lamiaceae essential oils. Molecules 2009, 14, 4213–4230. [Google Scholar] [CrossRef] [Green Version]
- Roller, S.; Ernest, N.; Buckle, J. The antimicrobial activity of high-necrodane and other lavender oils on methicillin-sensitive and -resistant Staphylococcus aureus (MSSA and MRSA). J. Altern. Complement. Med. 2009, 15, 275–279. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Łopusiewicz, Ł.; Kostek, M.; Drozłowska, E.; Pruss, A.; Wojciuk, B.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Dołęgowska, B. The Antibacterial Activity of Lavender Essential Oil Alone and in Combination with Octenidine Dihydrochloride against MRSA Strains. Molecules 2019, 1, 95. [Google Scholar] [CrossRef] [Green Version]
- Padiyara, P.; Inoue, H.; Sprenger, M. Global Governance Mechanisms to Address Antimicrobial Resistance. Infect. Dis. 2018, 11, 1178633718767887. [Google Scholar] [CrossRef] [Green Version]
- Todorova, D.; Yavorov, N.; Lasheva, V.; Damyanova, S.; Kostova, I. Lavender Essential Oil as Antibacterial Treatment for Packaging Paper. Coatings 2023, 13, 32. [Google Scholar] [CrossRef]
- Tkachenko, H.; Opryshko, M.; Gyrenko, O.; Maryniuk, M.; Buyun, L.; Kurhaluk, N. Antibacterial Properties of Commercial Lavender Essential Oil against Some Gram-Positive and Gram-Negative Bacteria. Agrobiodiversity Improv. Nutr. Health Life Qual. 2022, 6, 2. [Google Scholar] [CrossRef]
- Batiha, G.E.S.; Teibo, J.O.; Wasef, L.; Shaheen, H.M.; Akomolafe, A.P.; Teibo, T.K.A.; Al.-kuraishy, H.M.; Al.-Garbeeb, A.I.; Alexiou, A. A review of the bioactive components and pharmacological properties of Lavandula species. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 877–900. [Google Scholar] [CrossRef]
- Walasek-Janusz, M.; Grzegorczyk, A.; Zalewski, D.; Malm, A.; Gajcy, S.; Gruszecki, R. Variation in the Antimicrobial Activity of Essential Oils from Cultivars of Lavandula angustifolia and L. × intermedia. Agronomy 2022, 12, 2955. [Google Scholar] [CrossRef]
- Han, X.; Chen, Q.; Zhang, X.; Peng, J.; Zhang, M.; Zhong, Q. The elimination effects of lavender essential oil on Listeria monocytogenes biofilms developed at different temperatures and the induction of VBNC state. Lett. Appl. Microbiol. 2022, 74, 1016–1026. [Google Scholar] [CrossRef]
- Cavanagh, H.; Wilkinson, J. Biological activities of lavender essential oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef]
- Altun, M.; Yapici, B.M. Determination of chemical compositions and antibacterial effects of selected essential oils against human pathogenic strains. An. Acad. Bras. Cienc. 2022, 94, e20210074. [Google Scholar] [CrossRef]
- Hossain, S.; Heo, H.; De Silva, B.C.J.; Wimalasena, S.H.M.P.; Pathirana, H.N.K.S.; Heo, G.-J. Antibacterial activity of essential oil from lavender (Lavandula angustifolia) against pet turtle-borne pathogenic bacteria. Lab. Anim. Res. 2017, 33, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Adaszyńska-Skwirzyńska, M.; Zych, S.; Szczerbińska, D. In Vitro antibacterial effect of enrofloxacin combined with lavender essential oil on selected Salmonella serotypes isolated most commonly in poultry. Med. Wet. 2023, 79, 182–185. [Google Scholar] [CrossRef]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 6, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a027029. [Google Scholar] [CrossRef] [Green Version]
- Garneau-Tsodikova, S.; Labby, K.J. Mechanisms of Resistance to Aminoglycoside Antibiotics: Overview and Perspectives. Medchemcomm 2016, 1, 11–27. [Google Scholar] [CrossRef] [Green Version]
- Becker, B.; Cooper, M.A. Aminoglycoside antibiotics in the 21st century. ACS Chem. Biol. 2013, 1, 105–115. [Google Scholar] [CrossRef]
- Adaszyńska-Skwirzyńska, M.; Dzięcioł, M.; Szczerbińska, D. Lavandula angustifolia Essential Oils as Effective Enhancers of Fluconazole Antifungal Activity against Candida albicans. Molecules 2023, 28, 1176. [Google Scholar] [CrossRef]
- van Vuuren, S.; Viljoen, A. Plant-based antimicrobial studies-methods and approaches to study the interaction between natural products. Planta Med. 2011, 11, 1168–1182. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- Zhang, F.; Cheng, W. The Mechanism of Bacterial Resistance and Potential Bacteriostatic Strategies. Antibiotics 2022, 11, 1215. [Google Scholar] [CrossRef]
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res. 2018, 1, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Chellat, M.F.; Raguž, L.; Riedl, R. Targeting Antibiotic Resistance. Angew. Chem. Int. Ed. Engl. 2016, 23, 6600–6626. [Google Scholar] [CrossRef]
- Hershberg, R. Antibiotic-Independent Adaptive Effects of Antibiotic Resistance Mutations. Trends Genet. 2017, 8, 521–528. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 464–473. [Google Scholar] [CrossRef] [Green Version]
- Polyzou, A.; Slavakis, A.; Pournaras, S.; Maniatis, A.N.; Sofianou, D.; Tsakris, A. Predominance of a methicillin-resistant Staphylococcus aureus clone susceptible to erythromycin and several other non-β-lactam antibiotics in a greek hospital. J. Antimicrob. Chemother. 2001, 48, 231–234. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 13, 2471. [Google Scholar] [CrossRef] [Green Version]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- de Groot, A.C.; Schmidt, E. Tea tree oil: Contact allergy and chemical composition. Contact Dermat. 2016, 75, 129–143. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Meng, X.; Li, Y.; Zhao, C.N.; Tang, G.Y.; Li, H.B. Antibacterial and Antifungal Activities of Spices. Int. J. Mol. Sci. 2017, 18, 1283. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Kim, D.S.; Park, S.H.; Park, H. Phytochemistry and Applications of Cinnamomum camphora Essential Oils. Molecules 2022, 27, 2695. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, Y.; Fu, C.; Yang, H.; Liu, X.; Qiu, F.; Wang, X.; Wang, Z. Chemical Variation and Environmental Influence on Essential Oil of Cinnamomum camphora. Molecules 2023, 28, 973. [Google Scholar] [CrossRef]
- Szewczuk, M.A.; Zych, S.; Oster, N.; Karakulska, J. Activity of Patchouli and Tea Tree Essential Oils against Staphylococci Isolated from Pyoderma in Dogs and Their Synergistic Potential with Gentamicin and Enrofloxacin. Animals 2023, 13, 1279. [Google Scholar] [CrossRef]
- Djenane, D.; Yanguela, J.; Roncales, P.; Aider, M. Use of essential oils as natural food preservatives: Effect on the growth of Salmonella enteritidis in liquid whole eggs stored underabuse refrigerated conditions. J. Food Res. 2013, 2, 65–78. [Google Scholar] [CrossRef]
- Thosar, N.; Basak, S.; Bahadure, R.N.; Rajurkar, M. Antimicrobial efficacy of five essential oils against oral pathogens: An in vitro study. Eur. J. Dent. 2013, 7, 71–77. [Google Scholar] [CrossRef] [Green Version]
- De Rapper, S.; Kamatou, G.; Viljoen, A.; van Vuuren, S. The in vitro antimicrobial activity of Lavandula angustifolia essential oil in combination with other aroma-therapeutic oils. J. Evid. Based Complement. Altern. Med. 2013, 2013, 852049. [Google Scholar]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 3, 318–327. [Google Scholar] [CrossRef]
- Rosato, A.; Piarulli, M.; Corbo, F.; Muraglia, M.; Carone, A.; Vitali, M.E.; Vitali, C. In Vitro synergistic action of certain combinations of gentamicin and essential oils. Curr. Med. Chem. 2010, 17, 3289–3295. [Google Scholar] [CrossRef]
- Moussaoui, F.; Alaoui, F. Evaluation of antibacterial activity and synergistic effect between antibiotic and the essential oils of some medicinal plants. Asian Pac. J. Trop. Biomed. 2016, 6, 32–37. [Google Scholar] [CrossRef] [Green Version]
- Ismail, M.; Kemegne, G.A.; Njayou, F.N.; Penlap, V.; Mbacham, W.F.; Kamdem, S.L.S. Chemical composition, antibiotic promotion and in vivo toxicity of Piper nigrum and Syzygium aromaticum essential oil. Afri. J. Biochem. Res. 2017, 11, 58–71. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 5th ed.; CLSI supplement VET01S: Wayne, PA, USA, 2020. [Google Scholar]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST). Terminology Relating to Methods for the Determination of Susceptibility of Bacteria to Antimicrobial Agents. In Eucast Definitive Document E.Def. 1.2.; European Society of Clinical Microbiology and Infectious Diseases (ESCMID): Basel, Switzerland, 2002; Volume 6, pp. 503–508. [Google Scholar]
| Antibacterial Agent | Blue River | Ellagance Purple | Type of Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC0 | MICc | FIC | FICI | MIC0 | MICc | FIC | FICI | ||
| Staphylococcus aureus ATCC 25923 | |||||||||
| Essential oil (% v/v) | 0.125 | 0.016 | 0.128 | 0.192 | 0.250 | 0.008 | 0.032 | 0.288 | synergistic |
| Gentamicin (µg/mL) | 0.250 | 0.016 | 0.064 | 0.125 | 0.032 | 0.256 | |||
| Staphylococcus aureus MRSA | |||||||||
| Essential oil (% v/v) | 0.250 | 0.032 | 0.128 | 0.191 | 1.250 | 0.016 | 0.013 | 0.263 | synergistic |
| Gentamicin (µg/mL) | 16.0 | 1.0 | 0.063 | 16.0 | 4.0 | 0.250 | |||
| Pseudomonas aeruginosa ATCC 9027 | |||||||||
| Essential oil (% v/v) | 0.750 | 0.625 | 0.833 | 1.083 | 2.0 | 1.500 | 0.750 | 1.250 | no interaction |
| Gentamicin (µg/mL) | 2.0 | 0.500 | 0.250 | 2.0 | 1.0 | 0.5 | |||
| Antibacterial Agent | Blue River | Ellagance Purple | Type of Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC0 | MICc | FIC | FICI | MIC0 | MICc | FIC | FICI | ||
| Staphylococcus aureus ATCC 25923 | |||||||||
| Essential oil (% v/v) | 0.500 | 0.032 | 0.164 | 0.192 | 0.250 | 0.016 | 0.064 | 0.320 | synergistic |
| Gentamicin (µg/mL) | 0.125 | 0.016 | 0.128 | 0.125 | 0.032 | 0.256 | |||
| Staphylococcus aureus MRSA | |||||||||
| Essential oil (% v/v) | 1.250 | 0.016 | 0.013 | 0.076 | 1.250 | 0.032 | 0.026 | 0.089 | synergistic |
| Gentamicin (µg/mL) | 32.0 | 2.0 | 0.063 | 32.0 | 2.0 | 0.063 | |||
| Pseudomonas aeruginosa ATCC 9027 | |||||||||
| Essential oil (% v/v) | 1.50 | 1.0 | 0.666 | 1.166 | 2.5 | 2.0 | 0.80 | 1.300 | no interaction |
| Gentamicin (µg/mL) | 2.0 | 1.0 | 0.500 | 2.0 | 1.0 | 0.50 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adaszyńska-Skwirzyńska, M.; Zych, S.; Bucław, M.; Majewska, D.; Dzięcioł, M.; Szczerbińska, D. Evaluation of the Antibacterial Activity of Gentamicin in Combination with Essential Oils Isolated from Different Cultivars and Morphological Parts of Lavender (Lavandula angustifolia Mill.) against Selected Bacterial Strains. Molecules 2023, 28, 5781. https://doi.org/10.3390/molecules28155781
Adaszyńska-Skwirzyńska M, Zych S, Bucław M, Majewska D, Dzięcioł M, Szczerbińska D. Evaluation of the Antibacterial Activity of Gentamicin in Combination with Essential Oils Isolated from Different Cultivars and Morphological Parts of Lavender (Lavandula angustifolia Mill.) against Selected Bacterial Strains. Molecules. 2023; 28(15):5781. https://doi.org/10.3390/molecules28155781
Chicago/Turabian StyleAdaszyńska-Skwirzyńska, Michalina, Sławomir Zych, Mateusz Bucław, Danuta Majewska, Małgorzata Dzięcioł, and Danuta Szczerbińska. 2023. "Evaluation of the Antibacterial Activity of Gentamicin in Combination with Essential Oils Isolated from Different Cultivars and Morphological Parts of Lavender (Lavandula angustifolia Mill.) against Selected Bacterial Strains" Molecules 28, no. 15: 5781. https://doi.org/10.3390/molecules28155781
APA StyleAdaszyńska-Skwirzyńska, M., Zych, S., Bucław, M., Majewska, D., Dzięcioł, M., & Szczerbińska, D. (2023). Evaluation of the Antibacterial Activity of Gentamicin in Combination with Essential Oils Isolated from Different Cultivars and Morphological Parts of Lavender (Lavandula angustifolia Mill.) against Selected Bacterial Strains. Molecules, 28(15), 5781. https://doi.org/10.3390/molecules28155781