Valorization of Prunus Seed Oils: Fatty Acids Composition and Oxidative Stability
Abstract
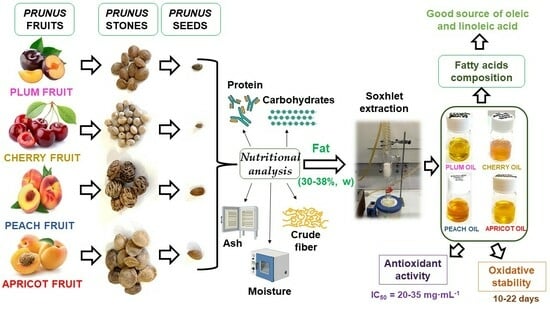
:1. Introduction
2. Results and Discussion
2.1. Evaluation of the Nutritional Analysis of Prunus Seeds
2.2. Evaluation of the Physico-Chemical Quality Characteristics of Prunus Seed Oils
2.2.1. Density Determination
2.2.2. Fatty Acid Composition
2.2.3. Lipid Health Quality Indexes
2.2.4. Antioxidant Activity
2.3. Comparative Study of the Oxidative Stability of Seed Oils
3. Materials and Methods
3.1. Reagents and Solvents
3.2. Sample Preparation
3.3. Nutritional Analysis of the Four Types of Fruit Seed of Prunus Family
3.3.1. Determination of Seed-to-Whole Stone Ratio
3.3.2. Determination of Moisture Content
3.3.3. Determination of Ash Content
3.3.4. Determination of Fat Content
3.3.5. Determination of Crude Fiber Content
3.3.6. Determination of Protein Nitrogen Content
3.3.7. Determination of Total Carbohydrates
3.4. Physico-Chemical Characterization of Prunus Seed Oils
3.4.1. Determination of Oil Density
3.4.2. Determination of Fatty Acid Profile
3.4.3. Lipid Nutritional Quality Indexes
3.4.4. Determination of the Antioxidant Capacity of Seed Oils
3.4.5. Evaluation of the Antioxidant Stability of Seed Oils
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Djilas, S.; Čanadanović-Brunet, J.; Ćetković, G. By-Products of Fruits Processing as a Source of Phytochemicals. Chem. Ind. Chem. Eng. Q. 2009, 15, 191–202. [Google Scholar] [CrossRef]
- Oreopoulou, V.; Tzia, C. Utilization of Plant By-Products for the Recovery of Proteins, Dietary Fibers, Antioxidants, and Colorants. Util. By-Prod. Treat. Waste Food Ind. 2007, 3, 209–232. [Google Scholar]
- Redondo, D.; Arias, E.; Oria, R.; Venturini, M.E. Thinned Stone Fruits Are a Source of Polyphenols and Antioxidant Compounds. J. Sci. Food Agric. 2017, 97, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, C.; Dhir, A.; Akram, M.U.; Salo, J. Food Loss and Waste in Food Supply Chains. A Systematic Literature Review and Framework Development Approach. J. Clean. Prod. 2021, 295, 126438. [Google Scholar] [CrossRef]
- Tsegaye, B.; Jaiswal, S.; Jaiswal, A.K. Food Waste Biorefinery: Pathway towards Circular Bioeconomy. Foods 2021, 10, 1174. [Google Scholar] [CrossRef]
- Mohamed, D.A.; Hamed, I.M.; Mohammed, S.E. Utilization of Grape and Apricot Fruits By-Products as Cheap Source for Biologically Active Compounds for Health Promotion. Egypt. J. Chem. 2021, 64, 2037–2045. [Google Scholar] [CrossRef]
- Lazos, E.S. Composition and Oil Characteristics of Apricot, Peach and Cherry Kernel. Grasas Aceites 1991, 42, 127–131. [Google Scholar] [CrossRef]
- Actualización, Ú. Boletín Fruta de Hueso. Resumen Campaña 2023 Inicio Producción Y Superficie Nacional-Avances ABRIL 2023. 2023. Available online: https://www.mapa.gob.es/es/agricultura/temas/producciones-agricolas/6_2023boletinfrutadehueso20234deoctubre2023_tcm30-661078.pdf (accessed on 10 July 2023).
- Natić, M.; Zagorac, D.D.; Ćirić, I.; Meland, M.; Rabrenović, B.; Akšić, M.F. Cold Pressed Oils from Genus Prunus. In Cold Press Oils: Green Technology, Bioactive Compounds, Functionality, and Applications; Academic Press: Cambridge, MA, USA, 2020; pp. 637–658. [Google Scholar] [CrossRef]
- Lara, M.V.; Bonghi, C.; Famiani, F.; Vizzotto, G.; Walker, R.P.; Drincovich, M.F. Stone Fruit as Biofactories of Phytochemicals With Potential Roles in Human Nutrition and Health. Front. Plant Sci. 2020, 11, 562252. [Google Scholar] [CrossRef]
- Rudke, C.R.M.; Zielinski, A.A.F.; Ferreira, S.R.S. From Biorefinery to Food Product Design: Peach (Prunus persica) By-Products Deserve Attention. Food Bioprocess Technol. 2022, 16, 1197–1215. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Rudzińska, M.; Raczyk, M.; Mišina, I.; Soliven, A.; Segliņa, D. Composition of Bioactive Compounds in Kernel Oils Recovered from Sour Cherry (Prunus cerasus L.) by-Products: Impact of the Cultivar on Potential Applications. Ind. Crops Prod. 2016, 82, 44–50. [Google Scholar] [CrossRef]
- Fidelis, M.; De Moura, C.; Kabbas, T.; Pap, N.; Mattila, P.; Mäkinen, S.; Putnik, P.; Kovačević, D.B.; Tian, Y.; Yang, B.; et al. Fruit Seeds as Sources of Bioactive Compounds: Sustainable Production of High Value-Added Ingredients from by-Products within Circular Economy. Molecules 2019, 24, 3854. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Radha; Kumar, M.; Puri, S.; Zhang, B.; Rais, N.; Pundir, A.; Chandran, D.; Raman, P.; Dhumal, S.; et al. Peach (Prunus persica (L.) Batsch) Seeds and Kernels as Potential Plant-Based Functional Food Ingredients: A Review of Bioactive Compounds and Health-Promoting Activities. Food Biosci. 2023, 54, 102914. [Google Scholar] [CrossRef]
- Khalil, M.N.A.; Farghal, H.H.; Farag, M.A. Outgoing and Potential Trends of Composition, Health Benefits, Juice Production and Waste Management of the Multi-Faceted Grapefruit Citrus Χ Paradisi: A Comprehensive Review for Maximizing Its Value. Crit. Rev. Food Sci. Nutr. 2022, 62, 935–956. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.P.J.; Prasad, S.R.; Banerjee, R.; Agarwal, D.K.; Kulkarni, K.S.; Ramesh, K.V. Green Solvents and Technologies for Oil Extraction from Oilseeds. Chem. Cent. J. 2017, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Eikani, M.H.; Golmohammad, F.; Homami, S.S. Extraction of Pomegranate (Punica granatum L.) Seed Oil Using Superheated Hexane. Food Bioprod. Process 2012, 90, 32–36. [Google Scholar] [CrossRef]
- Maszewska, M.; Florowska, A.; Dłuzewska, E.; Wroniak, M.; Marciniak-Lukasiak, K.; Zbikowska, A. Oxidative Stability of Selected Edible Oils. Molecules 2018, 23, 1746. [Google Scholar] [CrossRef] [PubMed]
- Arbex, A.K.; Bizarro, V.R.; Santos, J.C.S.; Araújo, L.M.M.; de Jesus, A.L.C.; Fernandes, M.S.A.; Salles, M.M.; Rocha, D.R.T.W.; Marcadenti, A. The Impact of the Essential Fatty Acids (EFA) in Human Health. Open J. Endocr. Metab. Dis. 2015, 5, 98–104. [Google Scholar] [CrossRef]
- Kaseke, T.; Opara, U.L.; Fawole, O.A. Fatty Acid Composition, Bioactive Phytochemicals, Antioxidant Properties and Oxidative Stability of Edible Fruit Seed Oil: Effect of Preharvest and Processing Factors. Heliyon 2020, 6, e04962. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H.A.; Franklin, B.; Kris-Etherton, P.; Harris, W.S.; Howard, B.; et al. Diet and Lifestyle Recommendations Revision 2006: A Scientific Statement from the American Heart Association Nutrition Committee. Circulation 2006, 114, 82–96. [Google Scholar] [CrossRef]
- Parodi, P.W. Has the Association between Saturated Fatty Acids, Serum Cholesterol and Coronary Heart Disease Been over Emphasized? Int. Dairy J. 2009, 19, 345–361. [Google Scholar] [CrossRef]
- Hashempour-Baltork, F.; Torbati, M.; Azadmard-Damirchi, S.; Savage, G.P. Chemical, Rheological and Nutritional Characteristics of Sesame and Olive Oils Blended with Linseed Oil. Adv. Pharm. Bull. 2018, 8, 107–113. [Google Scholar] [CrossRef]
- Ahmad, N.; Manzoor, M.F.; Shabbir, U.; Ahmed, S.; Ismail, T.; Saeed, F.; Nisa, M.; Anjum, F.M.; Hussain, S. Health Lipid Indices and Physicochemical Properties of Dual Fortified Yogurt with Extruded Flaxseed Omega Fatty Acids and Fibers for Hypercholesterolemic Subjects. Food Sci. Nutr. 2020, 8, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Santos-Silva, J.; Mendes, I.A.; Portugal, P.V.; Bessa, R.J.B. Effect of Particle Size and Soybean Oil Supplementation on Growth Performance, Carcass and Meat Quality and Fatty Acid Composition of Intramuscular Lipids of Lambs. Livest. Prod. Sci. 2004, 90, 79–88. [Google Scholar] [CrossRef]
- Kaya, C.; Kola, O. Some Characteristics and Fatty Acids Composition of Wild Apricot (Prunus pseudoarmeniaca L.) Kernel Oil. Artic. Asian J. Chem. 2008, 20, 2597–2602. [Google Scholar]
- Stryjecka, M.; Michalak, M.; Cymerman, J.; Kiełtyka-Dadasiewicz, A. Comparative Assessment of Phytochemical Compounds and Antioxidant Properties of Kernel Oil from Eight Sour Cherry (Prunus cerasus L.) Cultivars. Molecules 2022, 27, 696. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Chung, H.; Chang, P.S.; Lee, J.H. Development of a Method Predicting the Oxidative Stability of Edible Oils Using 2,2-Diphenyl-1-Picrylhydrazyl (DPPH). Food Chem. 2007, 103, 662–669. [Google Scholar] [CrossRef]
- Grosshagauer, S.; Steinschaden, R.; Pignitter, M. Strategies to Increase the Oxidative Stability of Cold Pressed Oils. LWT 2019, 106, 72–77. [Google Scholar] [CrossRef]
- Szterk, A.; Roszko, M.; Sosińska, E.; Derewiaka, D.; Lewicki, P.P. Chemical Composition and Oxidative Stability of Selected Plant Oils. JAOCS J. Am. Oil Chem. Soc. 2010, 87, 637–645. [Google Scholar] [CrossRef]
- Bozan, B.; Temelli, F. Chemical Composition and Oxidative Stability of Flax, Safflower and Poppy Seed and Seed Oils. Bioresour. Technol. 2008, 99, 6354–6359. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Cuevas, L.; Castellano, G.; Torrens, F.; Raikos, V. Revealing the Relationship between Vegetable Oil Composition and Oxidative Stability: A Multifactorial Approach. J. Food Compos. Anal. 2018, 66, 221–229. [Google Scholar] [CrossRef]
- Fox, N.J.; Stachowiak, G.W. Vegetable Oil-Based Lubricants-A Review of Oxidation. Tribol. Int. 2007, 40, 1035–1046. [Google Scholar] [CrossRef]
- Wettasinghe, M.; Shahidi, F. Scavenging of Reactive-Oxygen Species and DPPH Free Radicals by Extracts of Borage and Evening Primrose Meals. Food Chem. 2000, 70, 17–26. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; Tiwari, B.K.; Butler, F. Stability and Degradation Kinetics of Bioactive Compounds and Colour in Strawberry Jam during Storage. Food Bioprocess Technol. 2011, 4, 1245–1252. [Google Scholar] [CrossRef]
- Al-Bachir, M. Compositions and Microbial Properties of Gamma Irradiated Apricot (Prunus armeniaca L.) Kernel. J. Stress Physiol. Biochem. 2021, 17, 79–87. [Google Scholar]
- Farag, M.A.; Bahaa Eldin, A.; Khalifa, I. Valorization and Extraction Optimization of Prunus Seeds for Food and Functional Food Applications: A Review with Further Perspectives. Food Chem. 2022, 388, 132955. [Google Scholar] [CrossRef]
- Fratianni, F.; d’Acierno, A.; Ombra, M.N.; Amato, G.; De Feo, V.; Ayala-Zavala, J.F.; Coppola, R.; Nazzaro, F. Fatty Acid Composition, Antioxidant, and in Vitro Anti-Inflammatory Activity of Five Cold-Pressed Prunus Seed Oils, and Their Anti-Biofilm Effect Against Pathogenic Bacteria. Front. Nutr. 2021, 8, 775751. [Google Scholar] [CrossRef] [PubMed]
- Lykke, A.M.; Gregersen, S.B.; Padonou, E.A.; Bassolé, I.H.N.; Dalsgaard, T.K. Potential of Unconventional Seed Oils and Fats from West African Trees: A Review of Fatty Acid Composition and Perspectives. Lipids 2021, 56, 357–390. [Google Scholar] [CrossRef] [PubMed]
- Grancieri, M.; Martino, H.S.D.; Gonzalez de Mejia, E. Chia Seed (Salvia hispanica L.) as a Source of Proteins and Bioactive Peptides with Health Benefits: A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 480–499. [Google Scholar] [CrossRef]
- Yangilar, F. The Application of Dietary Fibre in Food Industry: Structural Features, Effects on Health and Definition, Obtaining and Analysis of Dietary Fibre: A Review. J. Food Nutr. Res. 2013, 1, 13–23. [Google Scholar]
- Andreasen, M.F.; Landbo, A.K.; Christensen, L.P.; Hansen, A.; Meyer, A.S. Antioxidant Effects of Phenolic Rye (Secale cereale L.) Extracts, Monomeric Hydroxycinnamates, and Ferulic Acid Dehydrodimers on Human Low-Density Lipoproteins. J. Agric. Food Chem. 2001, 49, 4090–4096. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.H.; McDonald, A. Fiber: Forms and Functions. Nutr. Res. 1998, 18, 617–624. [Google Scholar] [CrossRef]
- Ashraf, C.M.; Iqbal, S.; Ahmed, D. Nutritional and Physicochemical Studies on Fruit Pulp, Seed and Shell of Indigenous Prunus persica. J. Med. Plants Res. 2011, 5, 3917–3921. [Google Scholar]
- Shariatifar, N.; Pourfard, I.M.; Khaniki, G.J.; Nabizadeh, R.; Akbarzadeh, A.; Nejad, A.S.M. Mineral Composition, Physico-Chemical Properties and Fatty Acids Profile of Prunus armeniaca Apricot Seed Oil. Asian J. Chem. 2017, 29, 2011–2015. [Google Scholar] [CrossRef]
- Usenik, V.; Fabčič, J.; Štampar, F. Sugars, Organic Acids, Phenolic Composition and Antioxidant Activity of Sweet Cherry (Prunus avium L.). Food Chem. 2008, 107, 185–192. [Google Scholar] [CrossRef]
- Shukla, R.K.; Prajapati, K.; Shukla, A.; Singh, R. Evaluation of Nutritive Value, Phytochemical Screening, Total Phenolic Content and in-Vitro Antioxidant Activity of the Seed of Prunus domestica L. Plant Sci. Today 2021, 8, 830–835. [Google Scholar] [CrossRef]
- El-Aal, M.H.A.; Hamza, M.A.; Rahma, E.H. In Vitro Digestibility, Physico-Chemical and Functional Properties of Apricot Kernel Proteins. Food Chem. 1986, 19, 197–211. [Google Scholar] [CrossRef]
- Savic, I.; Gajic, I.S.; Gajic, D. Physico-Chemical Properties and Oxidative Stability of Fixed Oil from Plum Seeds (Prunus domestica Linn.). Biomolecules 2020, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Neagu, A.-A.; Niţa, I.; Botez, E.; Geaca, S. A Physico-Chemical Study for Some Edible Oils Properties. Analele Univ. Ovidius Constanta Ser. Chim. 2014, 24, 121–126. [Google Scholar] [CrossRef]
- Lee, M.R.F.; Tweed, J.K.S.; Kim, E.J.; Scollan, N.D. Beef, Chicken and Lamb Fatty Acid Analysis—A Simplified Direct Bimethylation Procedure Using Freeze-Dried Material. Meat Sci. 2012, 92, 863–866. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Liu, O. Development and Validation of a GC-FID Method for Quantitative Analysis of Oleic Acid and Related Fatty Acids. J. Pharm. Anal. 2015, 5, 223–230. [Google Scholar] [CrossRef] [PubMed]
- ATİK, İ.; Şevik, R.; Karasu, S. Soğuk Press Kiraz (Prunus avium) Çekirdeği Yayının Fizikokimyasal Özellikleri, Yağ Asidi, Sterol, Tokoferol ve Fenolik Bileşen Karakterizasyonu. Eur. J. Sci. Technol. 2019, 17, 959–965. [Google Scholar] [CrossRef]
- Perifanova-Nemska, M.; Delinska, N.; Dimitrova, E. Chemical Characteristics of Soap with Using Plum Kernel Oil (Prunus domestica L.). In Proceedings of the 2022 8th International Conference on Energy Efficiency and Agricultural Engineering, EE and AE 2022, Ruse, Bulgaria, 30 June–2 July 2022; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2022. [Google Scholar]
- Sales-Campos, H.; Reis de Souza, P.; Crema Peghini, B.; Santana da Silva, J.; Ribeiro Cardoso, C. An Overview of the Modulatory Effects of Oleic Acid in Health and Disease. Mini-Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.H.; Crawford, M.A.; Reifen, R. Update on Alpha-Linolenic Acid. Nutr. Rev. 2008, 66, 326–332. [Google Scholar] [CrossRef]
- Djoussé, L.; Matthan, N.R.; Lichtenstein, A.H.; Gaziano, J.M. Red Blood Cell Membrane Concentration of Cis-Palmitoleic and Cis-Vaccenic Acids and Risk of Coronary Heart Disease. Am. J. Cardiol. 2012, 110, 539–544. [Google Scholar] [CrossRef] [PubMed]
- de Souza, C.O.; Valenzuela, C.A.; Baker, E.J.; Miles, E.A.; Rosa Neto, J.C.; Calder, P.C. Palmitoleic Acid Has Stronger Anti-Inflammatory Potential in Human Endothelial Cells Compared to Oleic and Palmitic Acids. Mol. Nutr. Food Res. 2018, 62, 322. [Google Scholar] [CrossRef] [PubMed]
- Stryjecka, M.; Kiełtyka-Dadasiewicz, A.; Michalak, M.; Rachoń, L.; Głowacka, A. Chemical Composition and Antioxidant Properties of Oils from the Seeds of Five Apricot (Prunus armeniaca L.) Cultivars. J. Oleo Sci. 2019, 68, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Saldaña y Hernández, M.I.; Gómez-Álvarez, R.; Rivera-Cruz, M.d.C.; Álvarez-Solís, J.D.; Pat-Fernández, J.M.; Ortiz-García, C.F. The Influence of Organic Fertilizers on the Chemical Properties of Soil and the Production of Alpinia purpurata. Cienc. Investig. Agrar. 2014, 41, 215–224. [Google Scholar] [CrossRef]
- Lutterodt, H.; Slavin, M.; Whent, M.; Turner, E.; Yu, L. Fatty Acid Composition, Oxidative Stability, Antioxidant and Antiproliferative Properties of Selected Cold-Pressed Grape Seed Oils and Flours. Food Chem. 2011, 128, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Rokosik, E.; Dwiecki, K.; Siger, A. Nutritional Quality and Phytochemical Contents of Cold Pressed Oil Obtained from Chia, Milk Thistle, Nigella, and White and Black Poppy Seeds. Grasas Aceites 2020, 71, e368. [Google Scholar] [CrossRef]
- Lamine, M.; Mliki, A.; Mlikia, A. Genetic Diversity and Salt Tolerance in Barley Germplasm: Physiological, Biochemical & Molecular Characterization View Project Citrus Waste Valorization View Project Nutritional Quality Perceptions through Fatty Acid Profiling, Health Lipid Indices and Antioxidant Potentialities. Open J. Nutr. Food Sci. 2021, 3, 1015. [Google Scholar]
- Nawirska-Olszańska, A.; Kita, A.; Biesiada, A.; Sokół-ŁȨtowska, A.; Kucharska, A.Z. Characteristics of Antioxidant Activity and Composition of Pumpkin Seed Oils in 12 Cultivars. Food Chem. 2013, 139, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Bhushan, B.; Rosales, A.; Turienzo, L.R.; Cortina, J.L. Valorisation Potential of Cabernet Grape Pomace for the Recovery of Polyphenols: Process Intensification, Optimisation and Study of Kinetics. Food Bioprod. Process 2018, 109, 74–85. [Google Scholar] [CrossRef]
- Nederal, S.; Škevin, D.; Kraljić, K.; Obranović, M.; Papeša, S.; Bataljaku, A. Chemical Composition and Oxidative Stability of Roasted and Cold Pressed Pumpkin Seed Oils. JAOCS J. Am. Oil Chem. Soc. 2012, 89, 1763–1770. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). AOAC Method 925.10 (Air Oven Method) for Moisture in Flour, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Association of Official Analytical chemists (AOAC). Official Methods of the AOAC, Methods 923.03, 920.85, 920.87, 978.10, 950.46, 17th ed.; AOAC international: Gaithersburg, MD, USA, 2003. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Method 960.39, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Horwitz, W. AOAC Official Method 962.09. Fiber (Crude) in Animal Feed and Pet Food, 16th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- AOAC. Official Method 920.87—Protein (Total) in Flour, Final Action, 17th ed.; AOAC International: Gaithersburg, MD, USA, 1995. [Google Scholar]
- Ayoola, P.; Adeyeye, A. Chemical Evaluation of Food Value of Groundnut (Arachi hypogaea) Seeds. Am. J. Food Nutr. 2012, 2, 55–57. [Google Scholar] [CrossRef]
- Asha, K.K.; Anandan, R.; Mathew, S.; Lakshmanan, P.T. Biochemical Profile of Oyster Crassostrea Madrasensis and Its Nutritional Attributes. Egypt. J. Aquat. Res. 2014, 40, 35–41. [Google Scholar] [CrossRef]




| Sample | Seed (%, w) | Moisture (%, w) | Ash (%, w) | Fat (%, w) | Crude Fiber (%, w) | Protein Nitrogen (%, w) | Carbohydrates (%, w) |
|---|---|---|---|---|---|---|---|
| Peach | 2.4 ± 0.3 a | 7.6 ± 0.4 a | 6.1 ± 0.1 a | 30 ± 3 a | 8.63 ± 0.02 a | 35 ± 2 a | 13 ± 3 a |
| Apricot | 12.4 ± 0.6 b | 5.8 ± 0.3 b | 4.8 ± 0.4 b | 38 ± 2 b | 7.49 ± 0.02 b | 28.7 ± 0.8 b | 15 ± 3 b |
| Plum | 9.5 ± 0.3 c | 4.7 ± 0.4 c | 2.16 ± 0.02 c | 37.4 ± 0.4 b | 21.4 ± 0.2 c | 16 ± 2 c | 18 ± 2 c |
| Cherry | 10.3 ± 0.5 d | 3.6 ± 0.1 d | 1.43 ± 0.02 d | 36.0 ± 0.2 b | 23.9 ± 0.6 d | 10.3 ± 0.7 d | 25 ± 1 d |
| Sample | Density (g·mL−1) |
|---|---|
| Peach oil | 0.896 ± 0.001 a |
| Apricot oil | 0.897 ± 0.003 a |
| Plum oil | 0.903 ± 0.002 b |
| Cherry oil | 0.917 ± 0.005 c |
| Fatty Acids | Peach Oil (%) | Apricot Oil (%) | Plum Oil (%) | Cherry Oil (%) |
|---|---|---|---|---|
| Palmitic acid (C16:0) | (7.95 ± 0.08) a | (6.36 ± 0.03) b | (5.71 ± 0.03) b | (8.1 ± 0.8) a |
| Palmitoleic acid (C16:1n7) | (0.579 ± 0.002) a | (1.10 ± 0.03) b | (0.81 ± 0.01) c | (0.39 ± 0.01) d |
| Margaric acid (C17:0) | (0.0612 ± 0.0008) a | (0.051 ± 0.002) b | (0.0529 ± 0.0001) a,b | (0.092 ± 0.007) c |
| Stearic acid (C18:0) | (1.401 ± 0.004) a | (1.27 ± 0.05) a | (2.86 ± 0.05) b | (3.8 ± 0.6) c |
| Cis-Vaccenic acid (C18:1n7c) | (1.302 ± 0.007) a | (1.863 ± 0.024) b | (1.185 ± 0.005) c | (0.71 ± 0.02) d |
| Oleic acid (C18:1n9c) | (52.9 ± 0.4) a | (49.6 ± 0.5) b | (72.7 ± 0.2) c | (48.6 ± 0.9) b |
| Linoleic acid (C18:2n6c) | (35.4 ± 0.3) a | (39.3 ± 0.5) b | (16.4 ± 0.2) c | (36.1 ± 0.9) a |
| α-Linolenic acid (C18:3n3) | (0.129 ± 0.006) a | (0.172 ± 0.008) b | (0.081 ± 0.001) c | (0.1063 ± 0.0003) d |
| Arachidic acid (C20:0) | (0.143 ± 0.006) a | (0.14 ± 0.01) a | (0.1952 ± 0.0004) a | (1.3 ± 0.3) b |
| Gondoic acid (C20:1n9) | (0.090 ± 0.002) a | (0.104 ± 0.005) b | (0.079 ± 0.002) c | (0.398 ± 0.001) d |
| Behenic acid (C22:0) | ND | ND | ND | (0.23 ± 0.04) |
| Lignoceric acid (C24:0) | ND | ND | ND | (0.18 ± 0.02) |
| (9.55 ± 0.09) a | (7.8 ± 0.1) a | (8.82 ± 0.02) a | (14 ± 2) b | |
| (90.45 ± 0.09) a | (92.2 ± 0.1) a | (91.18 ± 0.02) a | (86 ± 2) b | |
| (54.9 ± 0.4) a | (52.7 ± 0.6) b | (74.7 ± 0.2) c | (50.1 ± 0.9) d | |
| (35.5 ± 0.3) a | (39.5 ± 0.5) b | (16.5 ± 0.2) c | (36.2 ± 0.9) a | |
| PUFA/SFA ratio | (3.7198 ± 0.0001) a | (5.046 ± 0.003) b | (1.86 ± 0.03) a | (2.7 ± 0.4) c |
| Lipid Indexes | Peach Oil | Apricot Oil | Plum Oil | Cherry Oil |
|---|---|---|---|---|
| Desirable fatty acid (DFA) | (91.85 ± 0.09) a | (93.44 ± 0.5) a,b | (94.05 ± 0.03) b | (90 ± 1) c |
| Atherogenicity (AI) | (0.088 ± 0.001) a | (0.0691 ± 0.0004) b | (0.0626 ± 0.0003) b | (0.09 ± 0.01) a |
| Thrombogenicity (TI) | (0.207 ± 0.002) a | (0.166 ± 0.002) a | (0.188 ± 0.001) a | (0.28 ± 0.04) b |
| Hypocholesterolemic/Hypercholesterolemic (H/H) | (11.3 ± 0.1) a | (14.29 ± 0.08) b | (15.83 ± 0.06) b | (11 ± 1) a |
| n6/n3 fatty acid ratio | (275 ± 10) a | (228 ± 8) b | (201.5 ± 0.6) c | (339 ± 9) d |
| Oils | DPPH IC50 (mg·mL−1 of Oil) |
|---|---|
| Peach oil | (31 ± 3) a |
| Apricot oil | (21.2 ± 0.9) b |
| Plum oil | (20 ± 3) b |
| Cherry oil | (35 ± 4) a |
| Storage Time (h) | IC50 Peach Oil (mg·mL−1 of Oil) | IC50 Apricot Oil (mg·mL−1 of Oil) | IC50 Plum Oil (mg·mL−1 of Oil) | IC50 Cherry Oil (mg·mL−1 of Oil) |
|---|---|---|---|---|
| 24 (1 day) | (31 ± 3) a | (21.2 ± 0.9) a | (20 ± 3) a | (35 ± 4) a |
| 48 (2 days) | (37 ± 1) b | n.m. | (21.9 ± 0.7) a,b | n.m. |
| 72 (3 days) | n.m. | (28.7 ± 0.5) b | n.m. | (41.6 ± 0.2) a,b |
| 120 (5 days) | (37.8 ± 0.6) b | n.m. | n.m. | n.m. |
| 168 (7 days) | n.m. | n.m. | (25 ± 1) b | n.m. |
| 240 (10 days) | (39 ± 3) c | (57 ± 1) c | n.m. | (46 ± 1) b,c |
| 360 (15 days) | n.m. | n.m. | (26 ± 3) b | (51 ± 4) c |
| 528 (22 days) | n.m. | n.m. | (26.2 ± 0.3) b | n.m. |
| Prunus Seed Oil | Intercept (mg·mL−1) | Slope (h−1) | t1/2 (h) | R2 |
|---|---|---|---|---|
| Peach | (−0.012 ± 0.003) | (4.0 ± 0.4) | 58 (2 days) | 0.90 |
| Apricot | (−0.006 ± 0.001) | (4.2± 0.1) | 110 (4 days) | 1.00 |
| Plum | (−0.0004 ± 0.0001) | (4.0 ± 0.3) | 1732 (72 days) | 0.78 |
| Cherry | (−0.0057 ± 0.0001) | (3.4 ± 0.2) | 121 (5 days) | 0.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Blázquez, S.; Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; García-Sánchez, B.; Miranda, R. Valorization of Prunus Seed Oils: Fatty Acids Composition and Oxidative Stability. Molecules 2023, 28, 7045. https://doi.org/10.3390/molecules28207045
Rodríguez-Blázquez S, Gómez-Mejía E, Rosales-Conrado N, León-González ME, García-Sánchez B, Miranda R. Valorization of Prunus Seed Oils: Fatty Acids Composition and Oxidative Stability. Molecules. 2023; 28(20):7045. https://doi.org/10.3390/molecules28207045
Chicago/Turabian StyleRodríguez-Blázquez, Sandra, Esther Gómez-Mejía, Noelia Rosales-Conrado, María Eugenia León-González, Beatriz García-Sánchez, and Ruben Miranda. 2023. "Valorization of Prunus Seed Oils: Fatty Acids Composition and Oxidative Stability" Molecules 28, no. 20: 7045. https://doi.org/10.3390/molecules28207045
APA StyleRodríguez-Blázquez, S., Gómez-Mejía, E., Rosales-Conrado, N., León-González, M. E., García-Sánchez, B., & Miranda, R. (2023). Valorization of Prunus Seed Oils: Fatty Acids Composition and Oxidative Stability. Molecules, 28(20), 7045. https://doi.org/10.3390/molecules28207045